
Courses

Carbocation and Carbanion are terms used to describe organic chemical entities that have an electrical charge on a carbon atom. The primary distinction between carbocation and Carbanion is that carbocation has a positively charged carbon atom, whereas Carbanion contains a negatively charged carbon atom.
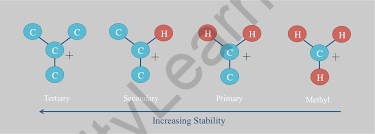
In contrast to carbocations and carbon radicals, electron-donating groups attached to the anionic core destabilize a carbanion because the centre already possesses an octet of electrons. As a result, the order of stability of carbanions is the inverse of that of carbocations and radicals.
Carbocations and carbanions are often encountered as intermediates in a variety of processes.
A carbanion is an organic species containing a negatively charged carbon atom, resulting from the removal of a proton (H⁺) from a carbon atom in a molecule. This negative charge makes the carbon atom electron-rich, and as a result, carbanions are highly reactive intermediates in organic chemistry. The general structure of a carbanion can be represented as , where can be hydrogen, alkyl, or aryl groups.
Carbanions are typically formed by the deprotonation of a carbon atom. This process can be induced under the influence of strong bases such as sodium hydride (NaH), lithium diisopropylamide (LDA), or alkoxides. The ease of carbanion formation depends on the acidity of the proton attached to the carbon atom. For example, molecules like acetylene, ethyl acetate, or compounds with electron-withdrawing groups (e.g., nitro, carbonyl) stabilize the negative charge, making their conjugate bases more likely to form carbanions.

An anion is a carbanion when the carbon is tervalent, makes three bonds, and has a formal negative charge in the one important mesomeric contribution. The stability is determined by the inductive hybridization impact of the anion's conjugation charge extent. The C-C of numerous bonds and neighbouring carbon atom lone pairs are two characteristics that influence Carbanion. A carbocation is positively charged, and as the number of positively charged atoms falls, so does the stability of carbon.
Electronegativity is an atom's capacity to attract electrons. The greater the Electronegativity, the greater the electron attraction to the atom. The Electronegativity of the positively charged carbon directly affects the stability of the carbocation. The carbocation's stability reduces as the carbon atom's Electronegativity rises. Sp> sp2 > sp3 The hybridization of the positively charged carbon in the vinylic carbocation is sp, which has a higher electronegativity than the hybridized carbon in the sp2 alkyl carbocation. As a result, a primary vinylic carbocation has lower stability than the main alkyl carbocation.