
Courses

By Shailendra Singh
|
Updated on 22 Nov 2024, 12:39 IST
Carbon tetrachloride is a man-made chemical that does not exist in nature. It is a clear liquid with a sweet odour that can be detected at low concentrations. Carbon chloride is also known as methane tetrachloride, perchloromethane, tetrachloroethane, and benziform. Carbon tetrachloride is a colourless gas that is commonly found in the atmosphere.
It is not flammable and does not dissolve easily in water. Earlier, it is being used to make refrigeration fluid and aerosol propellants, as a pesticide, a cleaning fluid and degreasing agent, fire extinguishers, and spot removers. By its negative effects, these applications are now prohibited, and it is only used in a few industrial applications.
In fact, carbon tetrachloride is a colourless, dense, highly toxic, volatile, non-flammable liquid with a distinctive odour that belongs to the family of organic halogen compounds and is primarily used in the production of dichlorodifluoromethane (a refrigerant and propellant).
Carbon tetrachloride, first produced in 1839 by the reaction of chloroform with chlorine, is now produced by the reaction of chlorine with carbon disulfide or methane. The methane process became dominant in the United States in the 1950s, but the carbon disulfide process remains important in countries where natural gas (the primary source of methane) is scarce.
Carbon tetrachloride has a boiling point of 77° C (171° F) and a freezing point of -23° C (-9° F); it is much denser than water and is practically insoluble in it.
This compound was previously widely used in cleaning agents. It was also used in fire extinguishers and was thought to be a precursor to a number of refrigerants. However, due to its toxicity, the use of this compound has been phased out by several governments. Inhaling large amounts of carbon tetrachloride can seriously harm vital organs such as the kidney and liver.
It can also be harmful to the central nervous system (CNS). Additionally, prolonged human exposure to carbon tetrachloride frequently results in death.
Carbon tetrachloride, as well known as tetrachloromethane, is an organic compound with the chemical formula CCl4. It is also known as carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR. This is a colourless liquid with a “sweet” odour that can be detected at low concentrations.

At lower temperatures, it is practically incombustible. It was previously widely used in fire extinguishers, as a refrigerant precursor, and as a cleaning agent, but it has since been phased out due to environmental and safety concerns. High levels of carbon tetrachloride (including vapour) can harm the central nervous system and degenerate the liver and kidneys.
Long-term exposure can be fatal.
The following are some of the most important physical and chemical properties of CCl4.
Despite the fact that tetrachloromethane has a wide range of applications, its use is highly restricted. Tetrachloromethane is a greenhouse gas that depletes the ozone layer and contributes significantly to environmental damage and global warming.
It is a volatile organic compound, but because it does not react, it has no effect on the formation of ground-level ozone. When tetrachloromethane is spilled on the soil, it quickly evaporates, though some is leached into the groundwater and causes contamination.
Tetrachloromethane, which causes ozone depletion, is extremely stable in the lower atmosphere, with a residence time of up to 30-50 years. Because of its chemical stability, it can reach the upper atmosphere, where it is subjected to intense ultraviolet radiation, which causes it to degrade to chlorine.
When this chlorine reacts with ozone, it depletes the ozone layer, which protects life from harmful ultraviolet radiation. It is a weak greenhouse gas that contributes significantly to global warming.
Tetrachloromethane does not pose a serious health risk in normal concentrations in the environment. It can cause severe damage to the liver, kidneys, and central nervous system at high levels. It may also cause cancer. Exposure to high levels of tetrachloromethane can result in coma or even death.
Aquatic organisms are also poisoned by tetrachloromethane.
Tetrahedral molecular geometries characterize carbon tetrachloride molecules, with the central carbon atom bonded to four chlorine atoms. The structure of CCl4 molecules is depicted in the diagram below.
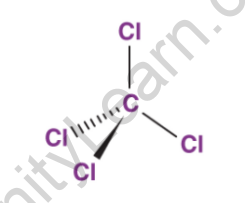
In fact, the four chlorine atoms present in the CCl4 molecule are symmetrically positioned at each corner around the central carbon atom. Covalent bonds exist between the carbon and chlorine atoms. This compound has non-polar properties due to its molecular geometry. It should be noted that the molecular structure of CCl4 is very similar to that of CH4 (methane gas).
Prior to the 1980s, the primary use of carbon tetrachloride was in the manufacture of chlorofluorocarbons for refrigeration. It has been used in fire extinguishers and as a cleaning agent.
Even so, because of the health risks associated with this compound, as well as the serious environmental damage caused by chlorofluorocarbons (see: ozone layer depletion), the use of this compound has been phased out by governments in several countries.
Moreover, this compound is known to have a variety of other applications, some of which are listed below.
Male Sprague-Dawley rats receiving a 500 mol/kg inhalation dose metabolise approximately 250 mol/kg tetrachloromethane. The determination of enzyme activities and histopathological evaluation show that for the same amount of tetrachloromethane metabolised under different oxygen partial pressures, various parameters reflecting cellular injury do not differ.
Under hypoxic conditions, however, the amounts of ethane and pentane exhaled are greater than under normoxia.
The previously reported differences in tetrachloromethane toxicity when exposed to normoxia or hypoxia appear to be primarily due to the different amounts of tetrachloromethane metabolised under both conditions.
Carbon tetrachloride was once widely used as a cleaning fluid (as a degreasing agent in dry cleaning institutions and other industries, and as a spot remover for clothes, furniture, and carpeting in households). It has been used to kill insects in fire extinguishers and as a fumigant in grain.
Water decomposes carbon tetrachloride in the presence of metals that can act as catalysts (such as iron and aluminium). Even in the absence of a metal catalyst, carbon tetrachloride can be decomposed to produce phosgene in the presence of overheated steam.
Humans inhaling carbon tetrachloride frequently experience negative short-term effects such as nausea, vomiting, lethargy, weakness, and headaches. This compound can also cause these symptoms if taken orally. Long-term CCl4 exposure is known to cause acute liver damage, acute kidney damage, and central nervous system damage. Carbon tetrachloride is widely regarded as a dangerous substance due to the health risks associated with it.