
Courses

By Shailendra Singh
|
Updated on 24 Jan 2025, 11:42 IST
Nuclear chemistry is a fascinating branch of chemistry that delves into changes occurring within the nuclei of atoms. These nuclear alterations form the basis for nuclear power, radioactivity, and a host of energy applications. The processes of nuclear fission and nuclear fusion are central to nuclear chemistry, each representing distinct mechanisms through which atomic nuclei release vast amounts of energy. This energy has implications in power generation, medicine, and scientific research.
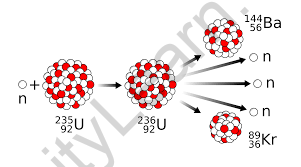
Nuclear fission is the process in which the nucleus of an atom splits into two or more smaller nuclei, along with the emission of neutrons and a significant release of energy. The process is typically triggered when a heavy atomic nucleus, such as uranium-235 or plutonium-239, absorbs a neutron. This absorption destabilizes the nucleus, causing it to split into smaller fragments known as fission products.
For example, when uranium-235 is bombarded with neutrons, the reaction produces krypton-94 and barium-139, accompanied by the emission of three neutrons and a massive amount of energy:
This chain reaction forms the foundation of energy production in nuclear reactors, where controlled fission generates electricity.
Nuclear fusion, in contrast, involves the joining of two light atomic nuclei to form a heavier nucleus. This process releases even more energy than fission. Fusion is the process that powers stars, including our sun, where immense pressure and temperatures enable hydrogen nuclei to combine and produce helium.
Mechanism of Nuclear Fusion
For example, fusion of deuterium (²H) and tritium (³H) occurs as follows:
This reaction releases significantly more energy than a comparable fission reaction.
Applications of Nuclear Fusion
The following table highlights key differences between nuclear fission and fusion:

| Aspect | Nuclear Fission | Nuclear Fusion |
| Process | Splitting of a heavy nucleus into smaller nuclei. | Combining of two light nuclei into a heavier nucleus. |
| Energy Release | High energy release, but less than fusion. | Extremely high energy release. |
| Raw Materials | Requires heavy isotopes like uranium-235 or plutonium-239. | Requires light isotopes like deuterium and tritium. |
| Conditions Needed | Initiated by low-energy neutrons. | Requires extremely high temperature and pressure. |
| Examples | Nuclear reactors, atomic bombs. | Sun’s energy, hydrogen bombs. |
| Waste | Produces radioactive waste. | Minimal radioactive waste. |
Both nuclear fission and fusion have transformative potential for energy production and other applications. While fission is currently the backbone of nuclear energy, fusion offers the promise of a cleaner, more abundant energy source for the future. Continued research and innovation are crucial to overcoming the challenges associated with both processes, paving the way for sustainable and efficient energy solutions.
Fission, as we describe it, is the splitting of an atom when it is struck by a highly active proton or neutron. Nuclear fusion, on the other hand, is the joining of two atoms into a single atom.
Nuclear chemistry, often known as radiochemistry, is the study of the elements that make up the cosmos, as well as the creation and production of radioactive pharmaceuticals for therapeutic purposes and a variety of other scientific applications.