
Courses

By Shailendra Singh
|
Updated on 22 Nov 2024, 13:06 IST
Hertz and Lenard’s observations of the photoelectric effect: Heinrich Hertz detected an unusual phenomenon while experimenting to confirm Maxwell’s electromagnetic theory of light in 1887. Hertz employed a spark gap to detect the presence of electromagnetic waves (two sharp electrodes arranged at a limited distance so that electric sparks can be created).
He placed it in a dark box to have a better look and discovered that the spark length had been shortened.
The spark length rose when he used a glass box and grew much more when he switched to a quartz box. The photoelectric effect was originally seen in this way. Wilhelm Hallwachs validated these findings a year later, demonstrating that UV light on a Zinc plate attached to a battery generated a current (because of electron emission).
J.J. Thompson discovered in 1898 that the amount of current flowing varied depending on the strength and frequency of the radiation employed.
The information about Hertz and Lenard’s observations of the photoelectric effect from various physics-related articles is available here. Hertz and Lenard’s observations of the photoelectric effect and its general concepts are important topics in physics.
Students who want to flourish in physics need to be well known about the photoelectric effect and Hertz and Lenard’s observations of the photoelectric effect to get deep knowledge about it to do well on their exams.
The experiments, brief explanation, and conclusions are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional physics help.
Lenard discovered in 1902 that the kinetic energy of released electrons increased with the frequency of radiation employed. This could not be explained because, according to Maxwell’s electromagnetic theory (which Hertz proved to be right), kinetic energy should be only dependent on light intensity (not frequency).

Einstein would also provide resolution a few years later when he provided an account for the photoelectric effect.
The oscillator constructed out of polished brass knobs coupled to induction coils and separated by a brief gap across which sparks might leap was used to test Maxwell’s Hertz hypothesis. Hertz built a basic looped wire receiver to verify what we’ve talked about. As we will see, its ends are separated by a little space.
The oscillator was located several yards away from the receiver. Hertz reasoned that if Maxwell’s predictions were right, electromagnetic waves would be sent during each sequence of sparks that would produce a current in the loop, which would then carry the sparks across the gap.
This usually happened when Hertz turned on the oscillator and produced the first electromagnetic wave receipt and transmission. Hertz further pointed out that an electrical wire reflects the waves, and that a concave reflector can focus them as well. He discovered that non-conductors allow the majority of the waves to pass through.
Heinrich Hertz discovered the phenomena of photoelectric emission while conducting electromagnetic wave experiments in 1887. He found that when light falls on a metal surface, some of the electrons near the surface absorb enough energy from the incident radiation to overcome the attraction of the positive ions in the surface material.
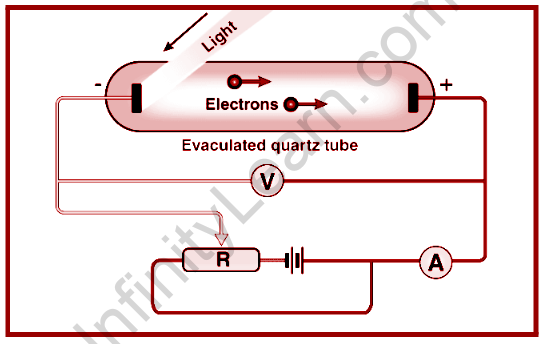

This observation, which is connected to the photoelectric effect and how this effect is verified by two scientists, is one of Heinrich Hertz’s experiments in 1887 to prove the theory of electromagnetic light when he saw peculiar events when he placed two electrodes at a very tiny distance.
So, we can claim that an electric spark can be formed, and he used a spark gap to conduct this experiment in order to conveniently observe the presence of electromagnetic waves. The same may be said of Lenard, who noticed the kinetic energy of electrons that are emitted and then increased with the radiation that was employed.
Since both their experiments show different types of samples or, to put it another way, both of their experiments illustrate the same thing, but they were all given green signals only after Einstein confirmed and proved it.
Kinetic energy is the energy that is possessed by a moving object or by its motion in a subject like physics. In other terms, it can be described as the work necessary by a body to accelerate from its current position to the desired position, and the body’s ability to maintain this kinetic energy until and unless it changes its speed.
The same is true in the event of speeding a body that is currently travelling at a rate that is causing it to deviate towards the rating part. It is believed that energy comes in various forms, including chemical energy, often known as thermal energy, electromagnetic energy, which includes nuclear energy and rest energy, and many others.
Thus, kinetic energy can be seen in both moving and stationary objects.
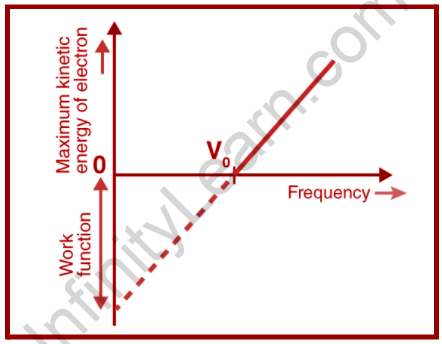
Each metal, as we’ve learned, has a minimum frequency of incident radiation below which no photoelectrons are emitted or are unable to emit; this frequency is referred to as the threshold frequency. The growing frequency of the incident beam keeps the number of photons unchanged, resulting in a proportionate rise in energy, as well as increasing the kinetic energy to its maximum, which leads to the voltage being stopped.
This is also commonly stated that when the intensity of incident radiation is increased, there will be no influence on the kinetic energy of photoelectrons because kinetic energy is determined by the frequency of light and thus the amount of energy delivered by each photon.
On the other hand, we can state that stopping the voltage is often provided with the light frequency that is to be fixed at a spot, rather than the intensity of the light.
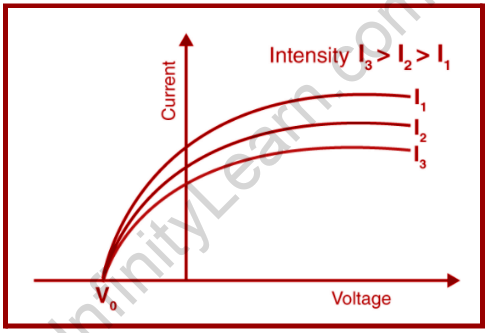
Heinrich Rudolf Hertz, a German physicist, established the Photoelectric Effect in 1887. In the context of all of this, we've talked about Hertz's work on radio waves. It was discovered that when ultraviolet light is shone on two metal electrodes with a voltage applied across them, the voltage at which sparking occurs varies.
The Photoelectric Effect is the result of ultraviolet radiation reducing the sparking voltage across a spark gap.
Our understanding of the quantum nature of light and electrons has grown as a result of research into the photoelectric effect. It has also affected the development of the wave-particle duality notion. The photoelectric effect is also commonly used to look at the energy levels of electrons in substances.
Photoconductive cells are semiconductor-based cells with bandgaps. These relate to the photon energies to be perceived. Such cells can be found in exposure metres and automatic street lamp switches that operate in the visible spectrum. Cadmium sulphide is used to make them. Infrared detectors, such as night-vision sensors, are instances of this. Mercury cadmium telluride or sulphur cadmium telluride are used to make them.