
Courses

By Shailendra Singh
|
Updated on 4 Dec 2024, 12:33 IST
Rutherford scattering, also known as the alpha particle scattering experiment, is a type of nuclear physics experiment. It was conducted on November 28, 1911, by Ernest Rutherford and Hans Geiger in the Cavendish Laboratory at the University of Cambridge. The experiment aimed to identify whether atoms have a positive or negative electric charge by measuring how often alpha particles (helium nuclei) are scattered in various directions when they collide with a thin gold foil. Rutherford scattering is one of the fundamental processes that can be used to determine atomic structure and chemical properties. Rutherford scattering was originally known as Coulomb scattering because it relies solely on the static electric (Coulomb) potential to determine the minimum distance between particles. Because neither the alpha particles nor the gold nuclei are internally excited, the classical Rutherford scattering process of alpha particles against gold nuclei is an example of “elastic scattering.” The Rutherford formula also ignores the massive target nucleus’ recoil kinetic energy.
Rutherford Atomic Model – J. J. Thomson’s plum pudding model failed to explain certain experimental results related to the atomic structure of elements. Ernest Rutherford, a British scientist, conducted an experiment and, based on his findings, proposed the atomic structure of elements, resulting in the Rutherford Atomic Model.
Rutherford’s experiment involves bombarding a thin sheet of gold with -particles and then studying the trajectory of these particles after they collided with the gold foil. In his experiment, Rutherford directed high-energy streams of -particles from a radioactive source at a thin sheet of gold (100 nm thickness). He wrapped a fluorescent zinc sulphide screen around the thin gold foil to study the deflection of the -particles. Certain observations made by Rutherford contradicted Thomson’s atomic model. Rutherford observed how alpha particles scattered from a thin gold foil after passing beams of alpha particles through it. The configuration of electrons within an atom interested Ernest Rutherford. Rutherford devised a test for this purpose. Fast-moving alpha ()-particles were made to fall on a thin gold foil in this experiment. Because he wanted the layer to be as thin as possible, he picked gold foil. The thickness of this gold foil was approximately 1000 atoms.
Particles are helium ions that are doubly charged. The fast-moving -particles have a lot of energy because they have a mass of 4. The particles were expected to be deflected by the subatomic particles in the gold atoms. He didn’t expect to see large deflections because the -particles were much heavier than the protons. However, the -particle scattering experiment produced completely unexpected results.
The intriguing results, however, revealed that approximately one in every 2000 alpha particles was deflected by very large angles (over 90°), while the rest passed through with little deflection. Rutherford deduced from this that the majority of the mass was concentrated in a small, positively-charged region (the nucleus) surrounded by electrons. When a (positive) alpha particle got close enough to the nucleus, it was repelled strongly enough to bounce at high angles. The small size of the nucleus accounted for the small number of alpha particles repelled in this manner. Rutherford demonstrated,
They used a thin gold foil with a thickness of 2.1×10-7 and placed it in the centre of a rotatable zinc sulphide detector and a microscope. They then fired a beam of 5.5MeV alpha particles from a radioactive source at the foil. As the alpha particles passed through the lead bricks, they were collimated. The scattering of these alpha particles after hitting the foil could be studied using the brief flashes on the screen. The results of this experiment were expected to teach Rutherford and his colleagues more about the structure of the atom.
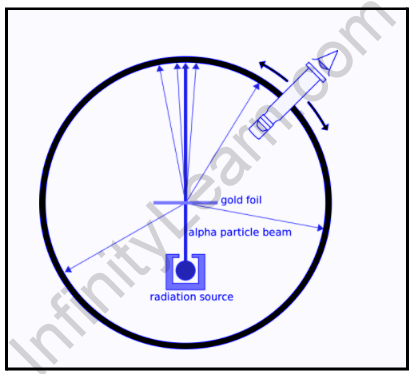

The results of this experiment did not agree with Thomson’s plum-pudding model of the atom. Rutherford concluded that because alpha particles are positively charged, they required a strong repelling force to be deflected back. He went on to argue that in order for this to happen, the atom’s positive charge must be concentrated in the centre, rather than scattered as in the previously accepted model. As a result, when the incident alpha particle came very close to the positive mass in the atom’s centre, it would repel, causing a deflection. However, if it passes through at a reasonable distance from this mass, there will be no deflection and it will simply pass through.
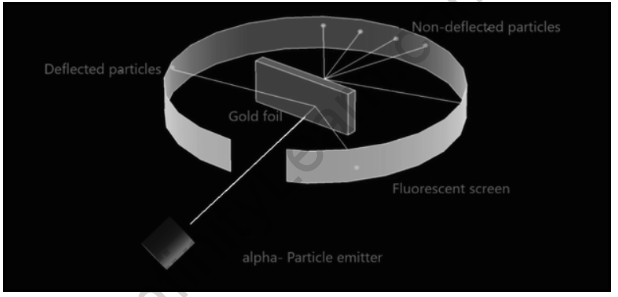
He conducts an experiment in which he bombards alpha particles into a thin sheet of gold and then observes their interaction with the gold foil as well as the trajectory or path taken by these particles. Rutherford conducts the experiment by passing very high streams of alpha-particles from a radioactive source, i.e. an alpha-particle emitter, through a thin sheet of gold with a thickness of 100 nm. He wrapped a screen of fluorescent zinc sulphide around the thin gold foil to examine the deflection caused by the alpha particles. Rutherford made some observations that contradict Thomson’s atomic model.
Rutherford’s Alpha Scattering Experiment yielded the following results:
First, he notices that the majority of the -particles bombarded towards the gold sheet pass through the foil without deflection, indicating that the majority of the space is empty. Some of the -particles were deflected through the gold sheet by very small angles, indicating that the positive charge in an atom is not distributed uniformly. In an atom, the positive charge is concentrated in a very small volume. Only a small percentage of the alpha-particles (1-2%) were deflected back, implying that only a small percentage of -particles had a nearly 180° angle of deflection. This demonstrates that the positively charged particles occupy a very small volume in comparison to the total volume of an atom.
Ernest Rutherford conducted an experiment involving the scattering of alpha particles. He bombarded the layered gold foil with positively charged alpha particles. He expected the alpha particles to revert back to the path they had taken when they were bombarded on the foil. However, the observed results were not the same. As a result, while the majority of the alpha particles pass through the foil, only a few particles experience small angle deflection.

Ernest Rutherford was interested in the arrangement of electrons in atoms. The fast-moving alpha particles were allowed to fall on the gold foil in the Rutherford experiment. The gold foil was hit with alpha particles. The thickness of this gold foil was approximately 1000 atoms. The alpha particles have a mass of four units and are doubly charged helium ions. This is a fast-moving particle that interacts with gold atom particles. Rutherford predicted that the subatomic particles in gold would revert the alpha particles. Because alpha particles are heavier than protons, it was expected that they would follow the same path as the protons. However, the observed results differed from what was expected.
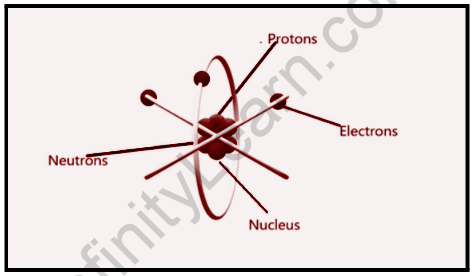
From this, Rutherford proposed a model of the atom with a positive charge at the center of mass, which he dubbed a ‘nucleus.’ The nucleus contains the entire mass of the atom. Negatively charged electrons orbit the positively charged nucleus in orbits.
The Rutherford Alpha Particle Scattering Experiment was conducted by Ernest Rutherford and his team to study the structure of the atom. They directed a beam of alpha particles at a thin gold foil and observed their scattering patterns to understand atomic structure.
The purpose of the experiment was to test the existing Thomson’s Plum Pudding Model of the atom and determine how atoms are structured internally.
The main apparatus included:
A thin gold foil (~1000 atoms thick).
A source of alpha particles (positively charged helium nuclei).
A circular fluorescent screen coated with zinc sulfide to detect scattered alpha particles.
Most alpha particles passed through the foil without deflection: Indicating that atoms are mostly empty space.
Some alpha particles were deflected at small angles: Suggesting that the positive charge is concentrated in a small region of the atom.