





Courses

By Shailendra Singh
|
Updated on 10 Feb 2025, 15:51 IST
What are Subshells? What is the difference between shells and subshells? An atom’s electrons are organized into shells that encircle the nucleus, for each succeeding shell moves further away from the nucleus. Subshells are made up of one or more atomic orbitals, while electron shells are made up of one or more subshells.
The energy of electrons in the same subshell is the same, whereas electrons in various shells or subshells possess different energies. Subshells are indeed a cluster of orbitals with the same principal quantum number or angular momentum quantum number, and shells are a series of subshells with the same principal quantum number.
The scattering of electrons in an element’s atomic orbitals is reflected by its electronic structure. Atomic electron arrangements follow the standard terminology whereby all electron-containing atomic subshells are placed in succession (with the number of electrons they possess written in superscript).
The primary quantum number represents the maximum number of electrons that can be contained in a shell (n). The shell number is expressed by the formula 2n2, where ‘n’ is the number of shells.
The azimuthal quantum number (denoted by ‘l’) dictates the subshells within which electrons are dispersed.
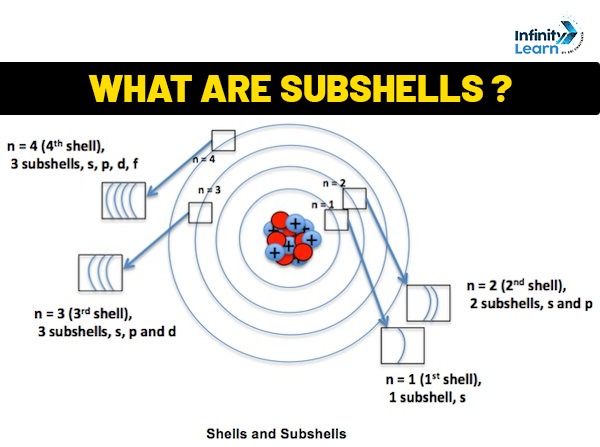
Concepts of shell and subshell
A shell is a path through which electrons traverse an atom’s nucleus. Because they are grouped all around the nucleus as per the energy of an electron in each shell, these shells are also known as energy ratios. The field of a shell through which electrons flow is known as a subshell. These are alluded to as angular momentum quantum numbers. The electron configuration of an element determines the allocation of electrons in its atomic orbitals. All electron-containing atomic subshells are grouped in a series in the notation of atomic electron compositions.

1p, 2d, and 3f orbitals are not conceivable since the azimuthal quantum number is always smaller than the main quantum number. As the principal quantum number ′n′ grows, so does the size of an orbital. To put it another way, the electron would be discovered outside of the nucleus.
Despite breakthroughs, incomprehension of the quantum-mechanical basis of electrons, shells, and subshells is still commonly used to describe electron configuration under the Bohr model of the atom.
An electron shell is the set of permissible states that electrons can inhabit that have the same primary quantum number, n (the number before the letter in the orbital label). The nth electron shell of an atom may hold 2n2 electrons; the first shell, for example, can have 2 electrons, the second shell 8 electrons, the third shell 18 electrons, and so on.
Because electron spin doubles the permissible states, each atomic orbital permits up to two identically equivalent electrons with opposite spins, one with a spin +12 (typically denoted by an up-arrow) and the other with a spin of 12 (commonly marked by a down-arrow) (with a down-arrow).
Within a shell, a subshell is the set of states described by a shared azimuthal quantum number, l. The value of l might be anywhere between 0 and n-1. The labels s, p, d, and f are represented by the numbers l = 0, 1, 2, 3. The 3d subshell, for example, has n = 3 and l = 2. 2(2l + 1) is the greatest number of electrons that can be deposited in a subshell.
This results in two electrons in the s subshell, six in the p subshell, ten in the d subshell, and fourteen in the f subshell. The quantum mechanics equations, especially the Pauli exclusion principle, stipulating that no two electrons in the same atom may have the same values as the four quantum numbers, determine the number of electrons that can occupy each shell and subshell.

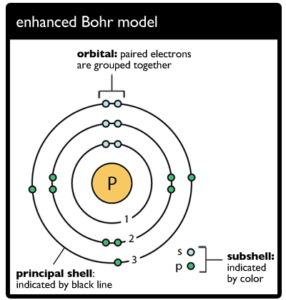
A valence shell that is not entirely covered with electrons or has not surrendered all of its valence electrons via chemical bonds with other atoms or molecules during such a chemical reaction is described as an open shell in the context of atomic orbitals. A filled valence shell, on the other hand, yields a closed shell. This setup is quite reliable. The term “open shell” refers to unpaired electrons in a molecule.
Learning the ideas in Chemistry would be easier if you prepare in a stage process and organized approach. The Structure of the Atom is the subject’s most challenging and high-scoring topic. This subject carries a lot of weight. Qualitative analysis might be the last resort because it takes so little time. Our solutions provide you with a thorough understanding of the conceptual themes.
The energy shell corresponds to a specific amount of energy. The greater the distance between the orbit and the nucleus, the more energy is connected with it. The energy level refers to these shells.
The first shell, or energy level, is the K shell; the second shell, L, is the second energy level; and the third shell, M, is the third energy level. The fourth shell and energy level is N.
The filling of electrons in shells progresses from lower to higher energy levels. Subshells with lower n + l values are filled first, followed by those with higher n + l values. When the n + l numbers are equal, the subshell with the lower n value is filled initially.