Table of Contents
Introduction
The transfer of charged species across the interface, specific adsorption of ions at the interface, and specific adsorption/orientation of polar molecules, including those of the solvent, all contribute to the appearance of electrode potential at the interface between an electrode and an electrolyte. The cathode and anode in an electrochemical cell each have their own electrode potential, and the difference between them is the cell potential. The electrode potential can be either that at equilibrium at the working electrode (“reversible potential”), potential with a non-zero net reaction but zero net currents on the working electrode or potential with a non-zero net current on the working electrode (like in galvanic corrosion or voltammetry). Extrapolation of measured values to the standard state can sometimes convert reversible potentials to the standard electrode potential for a given electroactive species.
Overview
Because electrode potentials are traditionally defined as reduction potentials, when calculating the overall cell potential, the sign of the potential for the metal electrode being oxidised must be reversed. Because the electrode potentials are independent of the number of electrons transferred (they are expressed in volts, which measure energy per electron transferred), the two electrode potentials can be simply added to give the overall cell potential even if the two electrode reactions involve different numbers of electrons.
Under non-equilibrium conditions, the electrode potential is determined by the nature and composition of the contacting phases, as well as the kinetics of electrode reactions at the interface (see Butler–Volmer equation). An operational assertion for determining electrode potentials with the standard hydrogen electrode involves this reference electrode in an ideal solution having “zero potential at all temperatures,” which is equivalent to the standard enthalpy of formation of hydrogen ion being “zero at all temperatures.”
Electrode potential
The electromotive force of a galvanic cell constructed from a standard reference electrode and another electrode to be characterised is referred to as electrode potential in electrochemistry. The standard hydrogen electrode is used as the reference electrode by convention (SHE) and it is defined as having a zero-volt potential. It is also known as the potential difference between charged metallic rods and salt solution.
The electrode potential is derived from the potential difference formed at the electrode-electrolyte interface. For example, the electrode potential of the M+/M redox couple is frequently mentioned.
Electrons flow from one chemical substance to another in an electrochemical process, propelled by an oxidation-reduction (redox) reaction. When electrons are transferred from an oxidised substance to a reduced substance, a redox reaction occurs. The substance that loses electrons and is oxidised in the process is known as the reductant, while the substance that gains electrons and is reduced in the process is known as the oxidant. The potential difference between the valence electrons in different elements determines the associated potential energy.
Since a reduction cannot occur without an oxidation and vice versa, a redox reaction can be described as two half-reactions, one representing the oxidation process and the other representing the reduction process.
Standard electrode potential
In general, the standard electrode potential is defined as “the value of the standard emf (electromotive force) of a cell in which molecular hydrogen is oxidised to solvated protons at the left-hand electrode under standard pressure.” It is a measure of an element’s or compound’s reducing power.
The foundation of an electrochemical cell, such as the galvanic cell, is always a redox reaction that can be divided into two half-reactions: oxidation at the anode (electronic loss) and reduction at the cathode (gain of an electron). The difference in electric potential between the two metal electrodes’ individual potentials with respect to the electrolyte generates electricity.
Even though the overall potential of a cell can be measured, measuring the electrode/electrolyte potentials in isolation is difficult. Temperature, concentration, and pressure all affect the electric potential. Because the oxidation potential of a half-reaction is the inverse of the reduction potential in a redox reaction, calculating either potential is sufficient. As a result, the standard electrode potential is also known as standard reduction potential. Metal ions from the solution have a tendency to deposit on the metal electrode at each electrode-electrolyte interface, attempting to charge it positively. Simultaneously, the electrode’s metal atoms have a tendency to dissolve as ions in the solution, leaving behind electrons that are attempting to negatively charge the electrode. At equilibrium, charges separate, and the electrode may be positively or negatively charged with respect to the solution, depending on the tendencies of the two opposing reactions. The electrode potential is the potential difference that develops between the electrode and the electrolyte. When the concentrations of all the species in a half-cell are equal, the electrode potential is referred to as standard electrode potential. Standard reduction potentials are now known as standard electrode potentials, according to IUPAC convention. The half-cell in a galvanic cell where oxidation occurs is known as the anode, and it has a negative potential with respect to the solution. The other half-cell in which reduction occurs is known as the cathode, and it has a positive potential in relation to the solution. As a result, there is a potential difference between the two electrodes, and when the switch is turned on, electrons flow from the negative electrode to the positive electrode. The current’s direction is the inverse of the direction of electron flow.
The electrode potential cannot be determined by trial and error. A pair of electrodes produce the galvanic cell potential. As a result, in a pair of electrodes, only one empirical value is available, and it is not possible to determine the value for each electrode in the pair using the empirically obtained galvanic cell potential.
Standard hydrogen electrode
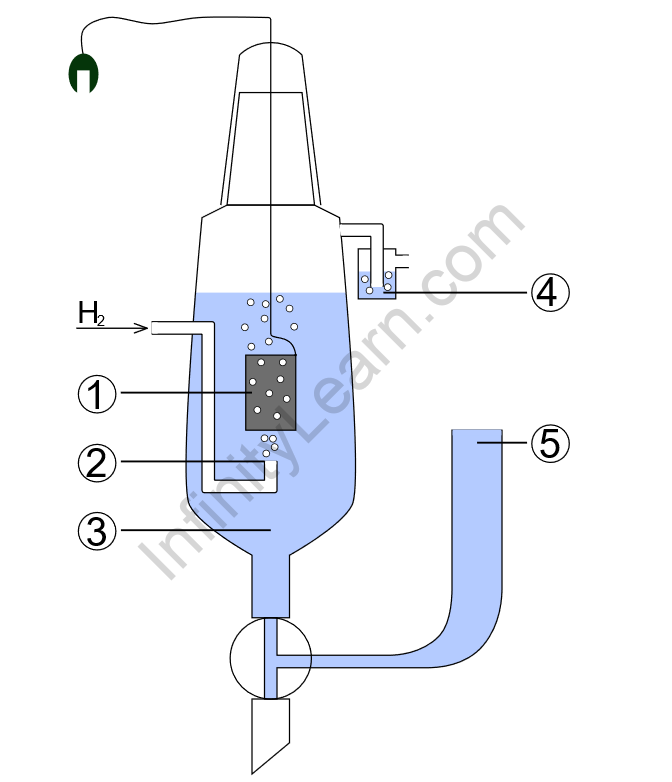
The standard hydrogen electrode (abbreviated SHE) is a redox electrode that serves as the foundation for the thermodynamic oxidation-reduction potential scale. In fact, the absolute electrode potential (E°) is estimated to be 4.44 ± 0.02 V at 25 °C, but for comparison with all other electro reactions, hydrogen’s standard electrode potential (E°) is considered as zero volts at any temperature. [1] At the same temperature, the potentials of any other electrodes are compared to those of the standard hydrogen electrode.
The redox half cell is the foundation of the hydrogen electrode:
2 H+(aq) + 2 e− → H2(g)
This redox reaction takes place at a platinum electrode that has been platinized. After dipping the electrode in an acidic solution, pure hydrogen gas is bubbled through it. The concentration of both the reduced and oxidised forms is kept constant at one. That means the pressure of hydrogen gas is one bar (100 kPa) and the activity coefficient of hydrogen ions in solution is one. Hydrogen ion activity is defined as their effective concentration, which is equal to the formal concentration multiplied by the activity coefficient. For very dilute water solutions, these unit-less activity coefficients are close to 1.00, but they are usually lower for more concentrated solutions.
FAQs
What is the standard hydrogen electrode?
Regular hydrogen electrode is a reference electrode to which all electrode potentials are calculated. Only when hydrogen gas is adsorbed at 1 atm over a platinum electrode dipped in 1 M HCl at 25oC, it is a regular electrode of hydrogen with a potential of E0=±0 volt.
What is the use of standard hydrogen electrodes?
SHE is the fundamental guide for reporting the capacity of quantitative half-cells. This is a kind of gas electrode that was commonly used in early studies as a reference electrode and an indicator electrode for calculating pH values.
What are the advantages of glass electrodes?
There seems to be a glass electrode that's extremely sensitive. Many types of smartphones use it. Cleaning and calibration with a common buffer solution are simple and straightforward. It covers both the acidic and alkaline pH ranges.






