Table of Contents
Classification of Aldoses and Ketoses
Aldoses:
- An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain, a carbonyl group on the endmost carbon atom, indicating that it is an aldehyde, and hydroxyl groups connected to all the other carbon atoms.
- Aldoses are distinguished from ketoses, which have the carbonyl group away from the molecule’s end and are thus ketones.
- Simple aldoses, like most carbohydrates, have the general chemical formula Cn(H2O)n. Because formaldehyde (n=1) and glycolaldehyde (n=2) are not considered carbohydrates, the simplest possible aldose is triose glyceraldehyde, which contains only three carbon atoms.
- All aldoses exhibit stereoisomerism because they have at least one asymmetric carbon center.
- Aldoses can exist in two forms: d- and l – The chirality of the asymmetric carbon furthest from the aldehyde end, namely the second-last carbon in the chain, is used to make the determination.
- Aldoses with alcohol groups on the right of the Fischer projection are called d-aldoses, while those with alcohol groups on the left are called l-aldoses. In nature, d-aldoses are more common than l-aldoses.
- Aldoses include glyceraldehyde, erythrose, ribose, glucose, and galactose. Seliwanoff’s test, which involves heating the sample with acid and resorcinol, can be used to chemically differentiate ketoses and aldoses.
- The test relies on the dehydration reaction, which occurs faster in ketoses, so that while aldoses react slowly, producing a light pink colour, ketoses react quickly and strongly, producing a dark red colour. The Lobry-de Bruyn-van Ekenstein transformation can convert aldoses to ketoses.
ketose:
- A ketose is a monosaccharide that contains one ketone group per molecule. With only three carbon atoms, dihydroxyacetone is the simplest ketose and the only one that has no optical activity. Because they can tautomerize into aldoses via an enediol intermediate and the resulting aldehyde group can be oxidised, all monosaccharide ketoses are reducing sugars, as demonstrated by the Tollens’ and Benedict’s tests.
- Nonreducing sugars are ketones that are bound into glycosides, such as the fructose moiety of sucrose.
Overview
Carbohydrates are the most abundant class of organic compounds, and they are the products of photosynthesis, which is formed by the endothermic reductive condensation of carbon dioxide in the presence of the pigment chlorophyll and light energy. They are the primary source of energy for metabolism for animals and plants, as well as living organisms that rely on plants for food. Carbohydrates with relatively small sizes are classified as sugars, and carbohydrates are further classified as aldoses and ketoses based on the C=O functional group.
Ketose and aldose are monosaccharides that can be distinguished by the group in which they are found. An aldose is a monosaccharide with an aldehyde group in its carbon skeleton. They’re mostly found in plants. Ketose is a monosaccharide with a ketone group on its carbon skeleton. They can only isomerize to aldose in the presence of reducing sugar. They’re found in processed foods. Ribulose, fructose, and other sugars are examples of ketose. To distinguish between aldose and ketose, we can use Seliwanoff’s Test.
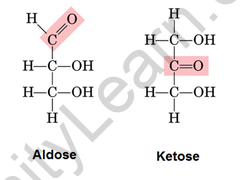
Difference between aldose and ketose
Carbohydrates must have an aldehyde group in order to be classified as aldose. Aldose is a monosaccharide with one aldehyde group per molecule.
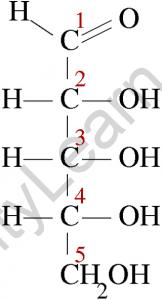
Diose glycolaldehyde is the most basic aldose, with only two carbon atoms. Because aldoses contain at least one asymmetric carbon centre, aldoses with three or more atoms exhibit the phenomenon of stereoisomerism.
Xylose, ribose, allose, lyxose, threose, glyceraldehyde, glucose, idose, galactose, talose, mannose, altrose, and arabinose are examples of aldoses. All of these aldoses have one aldehyde group in common, but they differ in the number of carbon atoms in their carbon skeleton.
Aldoses with stereogenic centres can exist in either L- or D-forms, and their form is determined by the chirality of the penultimate carbon.
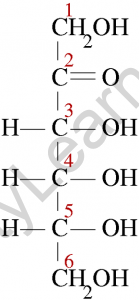
Ketose is a monosaccharide with the carbonyl function on the inner atoms of the carbon chain. Although dihydroxyacetone is not a sugar, it is a ketose analogue of glyceraldehyde. Commonly, a carbonyl group is attached at C-2, and the carbonyl function of ketoses can be reduced by sodium borohydride, yielding a mixture of epimeric products.
D-fructose is the sweetest of all-natural sugars, and its reduction yields a mixture of D-mannitol and D-glucitol (sorbitol), which are named after aldohexose and can also be obtained by the analogous reduction. Mannitol is also a naturally occurring carbohydrate. In general, ketoses are the distinct isomers of aldose monosaccharides. Because the chemistry of both classes is linked, the interconversion of aldoses and ketoses is simple in the presence of a base or acid catalyst.
The enediol tautomeric intermediate facilitates the corresponding epimerization and interconversion at the alpha sites of the carbon function.
Structure of Aldoses and Ketoses
Organic compound structures can be represented in a variety of ways. One of the most common methods is to employ Fischer projection formulas, which were developed by Nobel Prize-winning German chemist Emil Fischer.
Individual atoms are represented by their single-letter codes, and chemical bonds between them are represented by single, double, or triple dashes for single, double, and triple bonds, respectively, in these depictions of organic compounds drawn in a 2-dimensional field (on paper). These Fischer projection formulae are also commonly used to represent monosaccharides such as aldoses and ketoses.
The basic structure of an aldose is a carbon backbone with each carbon atom connected to its neighbouring carbon atom by a single bond. The aldehydic functional group is then attached to a carbon atom at each end of the backbone. The remaining carbon atoms are each bonded to one hydroxyl group (-OH) via a single bond.
The hydroxyl group attached to the carbon atom at one end of the backbone is referred to as a primary alcohol group, while the remaining hydroxyl groups are referred to as secondary alcohol groups. Bonding with hydrogen atoms fills the remaining valency of the carbon atoms.
The most common way to depict the chemical structures of ketoses, like aldoses, is to use Fischer projection formulae. The Fischer projection formula for Fructose, the most common ketose, with its functional group highlighted. Ketones, like aldoses, have a backbone composed of carbon atoms joined by a single covalent bond.
However, in ketoses, the carbonyl functional group is not attached to a carbon atom at either end of the chain.
As a result, the carbonyl carbon is linked to three different atoms: two carbon atoms (via two single bonds) and one oxygen atom (via a double bond). The remaining carbon atoms have hydroxyl groups attached to them, with the alcoholic groups at the backbone’s ends being primary alcohol groups.
FAQs:
What is the difference between an aldose and a ketose?
A monosaccharide with an aldehydic functional group as its main functional group is known as an aldose, whereas a ketose has a ketonic functional group as its main functional group. They're both polyhydroxy alcohols, but one is an aldehyde and the other is a ketone.
What exactly is a ketose example?
The six-carbon ketohexose fructose is one of the most common ketoses found in nature. It is most commonly found in many naturally occurring fruits as well as some plant foods such as honey and some vegetables.
What is the distinction between aldose and ketose?
The primary distinction between an aldose and a ketose is the type of functional group found in each. A ketose is a ketone, whereas an aldose is a functional aldehyde.






