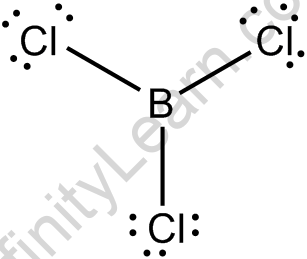Table of Contents
Boron trichloride is the inorganic compound with the recipe BCl3 . This drab gas is a reagent in natural amalgamation. It is profoundly receptive toward the water.
The kind of hybridization that happens in BCl3 is sp2 hybridization. In BCl3 particle, boron will be the focal iota which contains three reinforced iotas however no solitary pair of electrons. Its steric number is likewise supposed to Be3.
What do you mean by hybridization?
- The progressive idea of hybridization was presented by Scientist Pauling.
- It is characterized as the intermixing of two nuclear orbitals with comparative energy levels to give a deteriorated new type of orbitals. This intermixing depends upon quantum mechanics. Just the nuclear orbitals of comparable energy levels can take part in hybridization and both to some degree and completely filled can likewise take an interest in this interaction, given that they have comparable energy.
- The nuclear orbitals of comparative energy are combined as one during the course of hybridization, for example, the intermixing of 2 ‘s’ orbital or 2 ‘p’ orbital or intermixing of ‘s’ orbital with ‘p’ orbital or ‘s’ orbital with ‘d’ orbital.
How the hybridization happens in boron trichloride?
- To realize how the hybridization happens in boron trichloride we will take the focal molecule boron and check out at a couple of parts of it. We will take a gander at the ground condition of boron’s electron setup. It will be 1s2, 2s2, 2p1. To shape bonds with chlorine, boron will require three unpaired electrons. For this situation, one electron from the 2s is moved to the 2p level. Presently the electron design will be in an invigorated state and will be addressed as 1s2, 2s2, 2px1, 2py1.
- The hybridization of BCl3 currently happens where one 2s and two 2p orbitals of boron will participate in the process to frame three half-filled sp2 crossover orbitals. Each sp2 half and half orbitals will contain unpaired electrons that will cover with the unpaired electron in chlorine’s 3p orbital. Three σsp-p bonds are shaped between boron’s half-filled sp2 cross breed orbitals and three chlorine molecules.

Creation and structure
- Boron responds with incandescent light to give the comparing trihalides. Boron trichloride is, be that as it may, created economically by direct chlorination of boron oxide and carbon at 501 °C.
- The carbothermic response is similar to the Kroll cycle for the transformation of titanium dioxide to titanium tetrachloride. In the research facility BF3 responded with AlCl3 gives BCl3 through halogen exchange. BCl3 is a three-sided planar atom like the other boron trihalides, and has a bond length of 175pm.
- A level of π-holding has been proposed to clarify the short B− Cl distance in spite of the fact that there is some discussion with regards to its extent. It doesn’t dimerize, despite the fact that NMR investigations of combinations of boron trihalides shows the presence of blended halides. The shortfall of dimerisation diverges from the propensities of AlCl3 and GaCl3 , which structure dimers or polymers with 4 or 6 direction metal focuses.
BCl3 Lewis Structure
The BCl3 Lewis structure is like the BF₃( Boron Trifluoride), and BBr3 ( Boron Tribromide) as F and Br falls in bunch 7 and incorporates 7 valence electrons.
- Boron requires just 6 electrons rather than 8 to shape an octron.
- You can ascertain the conventional charges on the off chance that you are don’t know about that you have an ideal BCl3 Lewis Structure. You will analyze that B in BCl3 incorporates just 6 valence electrons.
- There are an aggregate of 24 valence electrons remembered for the BCl3 Lewis Structure.
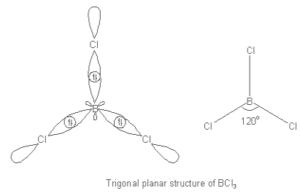
BCl3 Molecular Geometry and Bond Angles
- Assuming we analyze the design, we can observe that BCl3 sub-atomic math is three-sided planar and the BCl3 bond point is 120⁰. The focal particle atom is nonplanar and has a symmetric charge around it.
- The initial step to deciding the bond points of BCl3 is to frame the Lewis structure.
- For example, BCl3 is a three-sided planar and in this way it has a bond point of 120⁰. Notwithstanding, when a particle is polar, then, at that point, it can’t have bond points precisely of 120⁰ regardless of whether it is three-sided planar in shape.
- Another model we can take is CHICO, in such a case a bond point can’t go astray excessively far away from 120⁰ as it does exclude solitary pair electrons. Particles that remember a solitary pair for a focal electron will cause repugnance and that is the fundamental explanation for it.
- The BCl3 bond point will be under 120⁰ assuming the atom holds two holding gatherings and just one sets of electrons and causes a twisted sub-atomic shape. Because of solitary pair electrons, the atoms that have comparative space math can have particular sub-atomic calculations.
 FAQs
FAQs
Why is BCl3 sp2 hybridization?
Every chlorine particle utilizes its half-filled p-orbital for the σ-bond arrangement. Consequently the state of BCl3 is three-sided planar with bond points equivalent to 120°. Boron structures three σsp-p bonds with three chlorine particles by utilizing its half-filled sp2 crossover orbitals.
What type of bond is BCl3?
As B electrons in BCl3 go through sp2 hybridization, the resultant shape is three-sided planar. B and Cl structure polar covalent bonds, however three other covalent bonds have similar bond minutes. Accordingly the amount of three valence electrons emerges to be zero dipole second in a solitary bond.