Table of Contents
Introduction:
Acetylene (precise name: ethyne) is the synthetic compound with the recipe C2H2. It is a hydrocarbon and the least complex alkyne. This lackluster gas is broadly utilized as a fuel and a compound structure block. It is shaky in its unadulterated structure and in this way is typically dealt with as a solution. Pure acetylene is scentless, yet business grades for the most part have an obvious smell because of contaminations, for example, divinyl sulfide and phosphine.
As an alkyne, acetylene is unsaturated in light of the fact that its two carbon iotas are reinforced together in triple security. The carbon-carbon triple bond puts every one of the four iotas in a similar straight line, with CCH bond points of 180°.
Acetylene is likewise called Ethyne or Narcylen or Vinylene. It is generally utilized as a substance building block and as a fuel. In its unadulterated structure, it is unsteady is taken care of as an answer. It is an unsaturated compound the two carbon iotas in it are connected along with twofold security.
Vinylene is a boring gas that has a gentle ether-like scent. It is effectively dissolvable in water, chloroform, (CH3)2CO, and benzene. It is somewhat dissolvable in carbon disulfide and ethanol. It is lighter when contrasted with air and effectively touches off.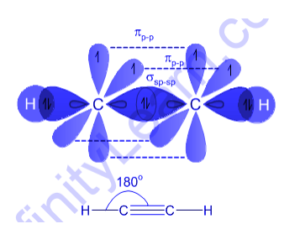
Hybridization of Ethyne (C2H2)
Whenever we separate ethyne atoms it essentially comprises of 2 CH particles. In any case, we will take first take both carbon and hydrogen particles independently and draw their orbital outlines. At the point when we do this, we will see that carbon has 6 electrons and hydrogen has one electron.
Presently, on the off chance that we see the electronic design of carbon in its ground state, it will be addressed as 1s2 2s2 2p2. At the point when it gets into an energized state, one of the electrons from 2s orbital will move or leap to the 2pz orbital and the electronic setup will change to 1s2 2s1 2px1 2py1 2pz1.
In the meantime, the CH particle has just 1 hydrogen iota, in this way the 2s1 and the 2pz1 orbitals get hybridized. This further prompts the arrangement of 4 sp hybridized orbitals wherein each CH particle will shape 2 hybridized sp orbitals. During hybridization, the C-C sigma bond is shaped when one sp orbital covers from every one of the carbons and two C-H bonds are made when a second sp orbital on every carbon covers with 1s orbital of hydrogen.
In this, the carbon molecule will have two half-filled 2p orbitals. These two sets of p orbitals don’t partake in the hybridization and on second thought structure two pi bonds bring about the production of a triple bond.
Equation and Structure of Acetylene
The synthetic equation of acetylene is C2H2, and its lengthy recipe is CH≡CH. The molar mass of acetylene can be given as 26.04 g/mol-1. In addition, this is the least complex alkyne particle, which is a useful gathering that is portrayed by researchers, as having triple bonds.
Its particles are direct 180 degrees, with its carbon iotas hybridized sp, therefore. Likewise, the two carbons have 2 sp orbitals, on which, one clings to the hydrogen and different bonds to the carbon basic bond. On the opposite side, the triple bond, that is 2 bonds that produce between the four P orbitals without hybridization, lies symmetrically to the straight framework.
Physical and Chemical properties
-
Changes of state:
At air pressure, acetylene can’t exist as a fluid and doesn’t have a liquefying point. The triple point on the stage graph compares to the softening point (−80.8 °C) at the negligible strain at which fluid acetylene can exist (1.27 atm). At temperatures beneath the triple point, strong acetylene can change straightforwardly to the fume (gas) by sublimation. The sublimation point at environmental tension is −84.0 °C.
-
Other:
At room temperature, the solvency of acetylene in (CH3)2CO is 27.9 g per kg. For a similar measure of dimethylformamide (DMF), the solvency is 51 g. At 20.26 bar, the solvency increments to 689.0 and 628.0 g for (CH3)2CO and DMF, individually. These solvents are utilized in compressed gas chambers.
-
Chemical property:
Acetylene is an exceptionally receptive substance compound inferable from its electrons in the C-C triple bond. For that reason, acetylene is a splendid nucleophile. Accordingly, it can experience a wide assortment of responses to get business items, like acrylic corrosive, acetylide, liquor, and a vinyl compound. We can likewise utilize it to get organometallic intensifies while responding with a metal like copper.
Uses of Acetylene
• Acetylene is utilized in brazing.
• Utilized in the glass business.
• Utilized in the assembling of manufactured elastic.
• Utilized in patching metals.
• Utilized as an added substance to safeguard food.
• Used to hasten metals.
• Used to produce acidic corrosive.
• Utilized as a feedstock in the assembling of acrylonitrile.
• Utilized in carburization of steel.
• Utilized as a fuel added substance.
Health Hazards Of C2H2
Individuals who interact with this compound might experience the ill effects of migraine, loss of cognizance, and wooziness. Passing because of gagging can happen on the off chance that a higher level of Ethyne is available in the air.
FAQs
What occurs assuming you breathe in acetylene?
Side effects of inward breath of acetylene incorporate discombobulation, weakness, sluggishness, queasiness, heaving, tachycardia and tachypnea. Openness to an elevated degree of acetylene will prompt a deficiency of awareness and passing. Acetylene is a boring gas generally utilized in welding processes.
How acetylene is created?
Acetylene is framed by any of three strategies: by the response of water with calcium carbide, by going through an electric curve of a hydrocarbon, or by halfway burning of methane with air or oxygen.
What is acetylene utilized for?
Acetylene is utilized in welding and cutting cycles. The welding system utilizing acetylene is known as cutting oxy-fuel or cutting coal. This strategy is utilized for cutting or welding materials that require temperatures of up to 3,500 ° C (6,330 ° F). Acetylene is fit for delivering the most sweltering fire of any remaining gases.





