Table of Contents
Important Topic of Chemistry: Polymerization
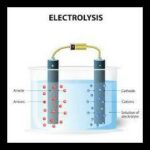
Water electrolysis is a well known technique utilized for various applications in different enterprises, for the most part in the food business, metallurgy, power plants among others. Additionally, the parts of water which incorporate hydrogen and oxygen have numerous applications. For example, hydrogen acquired through electrolysis is a perfect, sustainable and effective fuel source.
Water electrolysis is principally done to yield unadulterated hydrogen and oxygen gases. It includes passing an electric flow through the water which brings about the decay of water into hydrogen and oxygen.
The standard for Water Electrolysis
Two terminals or plates that are produced using a dormant metal, for example, platinum or iridium are put in the water. A DC electrical power source is associated with these plates. At the cathode (where electrons enter the water) part Hydrogen will show up. On the anode side, oxygen will show up. On the off chance that we consider the ideal faradaic proficiency, hydrogen will be delivered double how much oxygen. Then again, both will be relative to the complete electrical charge directed by the arrangement. Nonetheless, in certain cells side responses can happen and various items are framed with not so great faradaic productivity.
Electrolyte for Water Electrolysis
It is vital to pick the right electrolyte for water electrolysis. For what reason is it significant? On the off chance that we take a gander at the anion from the electrolyte it generally contends with the hydroxide particles to deliver an electron. Assuming that an electrolyte anion has a less standard anode potential than hydroxide it will be oxidized rather than the hydroxide. Accordingly, oxygen won’t be created. On account of a cation, assuming it has a more prominent standard cathode potential than a hydrogen particle it will be diminished. For this situation, hydrogen gas won’t be created.
Water Electrolysis in the Presence of Salts
Salts are 100%dissociate into cations and anions in water and henceforth increment the ionic focus for expanding conductivity. In any case, the cations and anions from the salt likewise will be drawn in towards the terminals and subsequently become contenders to the deterioration of water to create hydrogen and oxygen. Thus, the determination of salts with non-contending particles becomes vital.
Salts containing lesser standard terminal possibilities than hydrogen and hydroxide particles are appropriate for the electrolysis of water.
Particles of first and second gathering components (Li, Na, K, Mg, Ca, Ba, and so on) have lower standard potential than hydrogen particles and won’t be decreased and permit hydrogen particles from the water to hydrogen.
Non-responsive anions like nitrate, sulfate particles have lesser standard decrease potential than hydroxide particles. Sulfate oxidation to peroxy-sulfate has a decrease capability of +2.1V.
Non-solvent, strong polymeric ionic mixtures (Nafion), has been found to help electrolysis of water in under 1.5V.
Electrolysis of Pure Water
An abundance measure of energy as overpotential (to defeat different actuation obstructions) is generally expected for the electrolysis of unadulterated water. This abundance energy is critical in light of the fact that without it the cycle happens gradually and at times not under any condition. The restricted self-ionization of water is likewise a justification for this. Besides, the electrical conductivity of unadulterated water is around one-millionth than that of seawater. In such cases, the proficiency of electrolysis can be expanded by utilizing a legitimate electrolyte, for example, a salt, a corrosive or a base alongside electrocatalysts.
FAQs:
What occurs during water electrolysis?
Electrolysis of water is the interaction by which water is deteriorated into oxygen and hydrogen gas, when electric ebb and flow is gone through it. Water particle is decayed in to H+ and OH-particles, when electric momentum is gone through it.
Would you be able to do water electrolysis at home?
Break up salt or bake soft drink in water. By adding salt or baking soft drinks to the water, you increment the conduction of power through the water. Add some warm water to your glass and a tablespoon of salt or baking pop. Mix until the powder is completely broken up.



