Table of Contents
Introduction Fructose & Structure
Fructose is a sugar that can be found in fruits and vegetables. The only naturally occurring ketohexose is fructose (commonly known as fruit sugar). Because of its significantly levorotatory optical rotation, it’s also known as levulose. Fruit sugar, often known as fructose, is a ketonic simple sugar present in many plants, where it is frequently linked to glucose to produce the disaccharide sucrose. Along with glucose and galactose, it is one of three dietary monosaccharides that are directly absorbed into the bloodstream during digestion. Sugar cane, sugar beets, and maize are the commercial sources of fructose. The monosaccharides glucose and fructose are combined in high-fructose corn syrup. One molecule of glucose is covalently bonded to one molecule of fructose in the chemical sucrose. Fructose in all forms, including fruits and juices, is often used to improve the palatability and taste of foods and beverages, as well as to brown various dishes, such as baked products. The annual production of crystalline fructose is estimated to be around 240,000 tonnes.
Uses of Fructose’s
- It aids in the enhancement of the flavour of food and beverages.
- Crystalline fructose (natural sweetener) is found in a variety of beverages and low-calorie foods, including energy drinks, chocolate milk, cereals, and yoghurts.
- Products containing HFCS (High Fructose Corn Syrup) are a mixture of glucose and fructose.
- Many children’s and adult drugs, such as cough suppressants and decongestant liquids, contain high fructose corn syrup as a sweetener
Fructose’s Physical and Chemical Properties
- The carbohydrate can be fermented anaerobically using yeast or bacteria, resulting in the production of carbon dioxide and ethanol.
- In Maillard reactions, fruit sugar is employed because amino acids are present in an open-chain form, the interaction with glucose proceeds quickly.
- Hydroxymethylfurfural (‘HMF’) is formed when these chemicals dehydrate quickly.
It’s a crystalline white substance. - When compared to other sugars, these carbohydrates are extremely soluble.
- In comparison to other sugars, they absorb moisture swiftly and release it slowly into the environment.
Solubility and crystallisation of fructose
Fructose, like other sugars and sugar alcohols, has a higher water solubility. As a result, crystallising fructose from an aqueous solution is difficult. Because fructose is more soluble than other sugars, sugar mixtures containing fructose, such as sweets, are softer than sugar mixes including other sugars.
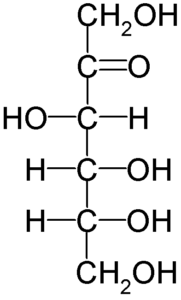
Fructose Structure
- The structure of fructose is cyclic.
- The development of the intramolecular hemiacetal is caused by the presence of the keto group.
- C5-OH joins with the ketonic group in the second position in this configuration.
- This results in the creation of chiral carbon and two CH2OH and OH group configurations.
- As a result, D-fructose has stereoisomerism, with the isomers -D-fructopyranose and -D-fructopyranose.
- It’s a crystalline white substance.
The Ring Closure Process (Hemiketal Synthesis)
The electrons on the alcohol oxygen are utilised to generate an ether by bonding the carbon in the second location.
The hydrogen is transferred to the carbonyl oxygen to form a new OH bond, giving the keto structure of fructose a ring
Hemiketal is a functional group of Hemiketal.
Anomeric carbon refers to the carbon at the centre of the hemiketal functional group. Both an ether oxygen and an alcohol group are connected to two additional carbons in the hemiketal group.
Fructose Alpha And Beta Structure Comparison
It’s a Beta structure if the alcoholic group (OH) is on the same side of the ring as the carbon at position 6. The alcoholic (OH) group at carbon 2 is projected upward due to the cyclic ring structure. If the –OH is on the same side of the ring as the carbon at 6, the ring structure causes the OH at carbon to project downward.
In the Chair Structures, Compare Glucose And Fructose
Fructose is a six-membered ring with a –OH group on the carbon at the fourth position in a down projection, whereas glucose is a six-membered ring with a –OH group on the carbon at the fourth position in a down projection.
Fructose Molecular and Structure Formula
Fructose is a levorotatory monosaccharide, meaning it rotates plane-polarized light to the left. Fructose has the molecular formula C6H12O6, although it has a different bonding pattern than glucose. Although fructose is a hexose, it exists as a five-member hemiketal ring. In comparison to glucose, the hemiketal ring aids a long metabolic pathway and high reactivity.
Also Get: NCERT Exemplar Solutions for Class 12 Chemistry – Free PDF Download
FAQs
What exactly is fructose?
Fructose is a monosaccharide, or single sugar, with a chemical formula similar to glucose but a distinct molecular structure. Fruit, some vegetables, honey, and other plants all contain fructose, sometimes known as fruit sugar. Carbohydrates, such as fructose and other sugars, provide the body with energy.
What structure does fructose have?
Fructose has a six-carbon linear chain with hydroxyl and carbonyl groups, just as all other simple sugars. However, most of it occurs as two hemiketal rings in crystalline form and in solution: -D-fructopyranose* (top) and -D-fructofuranose* (bottom) (bottom).







