Table of Contents
Preparation of Alcohol: All through the 10,000 years or so that humans have been drinking fermented beverages, they have also debated their benefits and drawbacks. The debate rages on to this day, with people debating whether alcohol is good or bad for them. It’s safe to say that alcohol is a tonic as well as a poison. The main difference is in the dosage. Small amounts of alcohol appears to be beneficial to the heart and circulatory system, and it may protect against type 2 diabetes and gallstones. In most countries, heavy drinking is a leading cause of preventable death. Alcohols seem to be organic compounds that contain one or more hydroxyl groups (–OH) that are bonded to aliphatic carbon atoms. ROH is the general formula for alcohol. Aromatic alcohols are alcohols that contain the hydroxyl group (–OH) in the side chain of an aromatic hydrocarbon. Alcohols are organic derivatives of water in which one of the hydrogen atoms has been replaced by an alkyl group, which is typically represented by the letter R in organic structures. In ethanol (or ethyl alcohol), for example, the alkyl group is the ethyl group.

Overview
Among the most common organic compounds are alcohols. They are used as sweeteners and perfume ingredients, as well as valuable intermediates in the synthesis of other compounds. They are also among the most abundantly produced organic chemicals in industry. In fact, ethanol and methanol are two of the most well-known alcohols (or methyl alcohol). Ethanol is used to sterilise hospital instruments, as well as in toiletries, pharmaceuticals, and fuels. This is also the one seen in alcoholic beverages. Ethanol is also used to make anaesthetic ether. Methanol is used as a solvent, a raw material in the production of formaldehyde and special resins, a special fuel, antifreeze, and for metal cleaning.
Steam distillation is a common method for isolating larger, more complex alcohols from plant volatile oils. The plant material is boiled in water, and the volatile oils are carried over by the steam and condensed before being separated from the water. Natural sources of alcohol include substances such as cholesterol, which is found in most animal tissues (and is abundant in egg yolks), and retinol (vitamin A alcohol), which is extracted from fish liver oils.
Preparation of alcohol
Alcohols are substances that have one or more hydroxyl (-OH) groups that are directly attached to a carbon chain. Alcohols in their free form are uncommon in nature; they are mostly found in essential or volatile oils extracted from plants’ flowers, leaves, and stems. Alcohols can be made by hydrating alkenes or reducing aldehydes, ketones, acids, and esters.
Method of preparation of alcohol
There are various methods for preparing alcohol:
- Oxymercuration and Demercuration of Alkanes:
Alkyl mercury compounds are formed when alkenes react with mercuric acetate in the presence of H2O and tetrahydrofuran. This is one of the most common methods of preparing alcohol.
- Preparation of Alcohols from Grignard Reagent:
Using Grignard reagents and carbonyl compounds, we can produce the three types of monohydric alcohols (primary, secondary, and tertiary alcohols). Alcohols are produced by the addition of RMgX to carbonyl compounds, followed by hydrolysis. Grignard reagent is said to be a kind of organometallic compound.
We can say that the Grignard reagent is extremely reactive. It reacts with a wide range of inorganic compounds, including water, carbon dioxide, and oxygen, as well as the majority of organic compounds. It’s worth noting that an alkane is such a weak acid that Grignard reagent can displace it with compounds that we’d normally consider to be very weak acids themselves, if not acids at all.
- Reduction of Carbonyl Compounds:
Humans can also obtain alcohol by reducing aldehydes and ketones. Aldehydes can be converted to primary alcohols and ketones to secondary alcohols. This can be accomplished through catalytic hydrogenation or through the use of chemical reducing agents such as lithium aluminium hydride, LiAlH4.
Of that kind reduction techniques are useful in the preparation of certain alcohols that are less available than their carbonyl counterparts. It is important to note that sodium borohydride, NaBH4, does not reduce carbon-carbon double bonds, even those conjugated with carbonyl groups.
- Reduction of Acids to Alcohols:
One of the few reagents that can convert acid to alcohol is lithium aluminium hydride or LiAlH4.
4RCOOH + 3LiAlH4 → 4RCH2OH 1oalcohol
LiAlH4 is a common ingredient in the laboratory for the reduction of not only acids but also many other classes of compounds due to its excellent yields.
- By the Action of Nitrous Acid on Primary Amines:
R-NH2 + HNO2 → R-OH + N2 + H2O
But, under similar conditions, CH3NH2 gives CH3-O-N=O or CH3OCH3
CH3NH2 + 2HNO2 → CH3-O-N=O + 2H2O + N2
OR 2CH3NH2 + 2HNO2 → CH3OCH3 + 2N2 + 3H2O
- By Fermentation:
Fermentation is the slow breakdown of complex organic compounds into simpler organic compounds by enzyme activity. Enzymes are non-living, complex, nitrogenous (proteins) macromolecules with a high molecular weight. These enzymes are typically obtained from living organisms.
This is usually followed by the evolution of gases such as CO2 and CH4. They produce a great deal of energy and are exothermic in nature. Yeast converts sugar into ethyl alcohol during the alcoholic fermentation process.
Also Read: Preparation of Aldehydes – From Alcohols and Hydrocarbons
Preparation of alcohol from alkyl halide
When alkyl halides are boiled with an alkali hydroxide aqueous solution, they give alcohol via a nucleophilic substitution mechanism.
R-X + KOH → R-OH + KX
This method generates both primary and secondary alcohols. Glycerol can be synthesised from propylene through a series of reactions, one of which is the hydrolysis of a halide.
Industrial preparation of alcohol
There seem to be three industrial methods for producing alcohol. All of these methods rely on raw materials derived from petroleum, natural gas, coal, and biomass.
In industries, starch is converted into ethyl alcohol using specific enzymes, a process known as fermentation. The starch is first degraded into a simpler form, and then glucose is converted to ethyl alcohol with the help of a specific enzyme.
For instance, ethanol is produced in large quantities by hydration of ethene under pressure at temperatures around 300o in the presence of phosphoric acid:
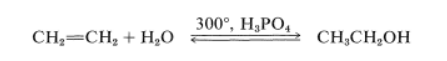
A soluble ethanol solution is obtained, which can be concentrated by distillation into a constant-boiling point mixture containing 95.6 percent ethanol by weight. Dehydration of the remaining few percent of water to produce “absolute alcohol” is accomplished either chemically or through benzene distillation, which results in preferential separation of the water. Ethanol is also produced in large quantities through fermentation, but this method is not competitive for industrial applications with ethene hydration. Hydration of the corresponding alkenes also produces isopropyl alcohol and tert-butyl alcohol.
FAQs
How is an alcohol prescription prepared?
The Grignard reagent is reacted with formaldehyde to produce a primary alcohol. Whenever a Grignard reagent is combined with another aldehyde, secondary alcohol is formed. At last, combining a Grignard reagent with a ketone results in the formation of a tertiary alcohol.
Which organism is required for the preparation of alcohol?
Yeasts are the principal fermentors and alcohol producers in the manufacture of wine, beer, and other alcoholic beverages. Saccharomyces cerevisiae is the most common yeast species used.
What is the fermentation process of alcohol?
Alcohol fermentation, as well known as ethanol fermentation, is an anaerobic yeast pathway that converts simple sugars to ethanol and carbon dioxide. Alcohol fermentation allows yeasts to break down sugar in the absence of oxygen, resulting in byproducts that humans can use.



