Table of Contents
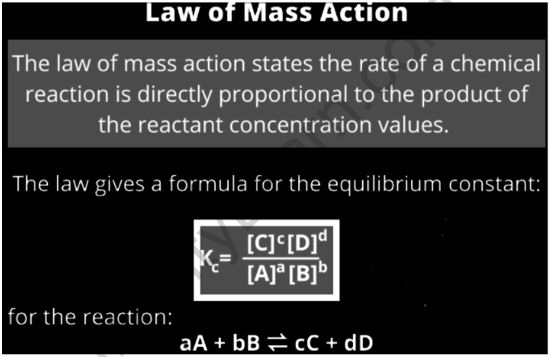
The law of mass action states that the rate of a chemical reaction is proportional to the product of the reactants’ activities or concentrations. It describes and forecasts how solutions in the dynamic system behave. It means that the ratio between the concentrations of reactants and products in a chemical reaction mixture that is in equilibrium is constant. In the initial formulation of the law, two aspects are involved: 1) the equilibrium aspect, which deals with the composition of a reaction mixture at equilibrium, and 2) the kinetic aspect, which deals with rate equations for elementary reactions.
A brief outline
Cato M. Guldberg and Peter Waage conducted research in which equilibrium constants were calculated using kinetic data and the rate equation. Chemical equilibrium, according to Guldberg and Waage, is a dynamic process in which the rates of forwarding and backward reactions must equal chemical equilibrium. The expression of the rate equation must be used to determine the expression of the equilibrium constant appealing to kinetics. The law is an equilibrium statement that gives an expression for the equilibrium constant, which is a variable that characterizes chemical equilibrium. This is calculated using equilibrium thermodynamics in modern chemistry. It can also be calculated using the chemical potential concept.
Important concepts
The Constant of Equilibrium (Kc)
At a particular temperature, the concentrations of reactants and products are constant when they are in equilibrium. Consider the simple reversible reaction below, in which A and B are the reactants and C and D are the products.
A + B ⇌ C + D
An equilibrium mixture is a mixture of products and reactants that is in a condition of chemical equilibrium. For an equilibrium mixture, there is a relationship between the concentration of products and the concentration of reactants. The following is an equivalence for this relationship:
Kc = [C][D]/[A][B]
Kc is referred to as the equilibrium constant in this case. The stoichiometric coefficients of the reactants and products are 1, and the concentration of A at equilibrium is written as [A] (similarly for B, C, and D). The equilibrium constant has been shown to be dependent on the stoichiometric coefficients of the reactants and products in experiments.
As a result, at a fixed temperature, the equilibrium constant is equal to the product of the product concentrations increased to their corresponding stoichiometric coefficients divided by the product of the concentration of reactants elevated to their appropriate stoichiometric coefficients. It is also described as the chemical equilibrium law or the equilibrium law.
The Law of Mass Action in Practice
Because this law also applies to semiconductors, it has a number of major consequences in the domains of electronics and semiconductor physics. When the semiconductor system is in thermal equilibrium, the law of mass action gives a link between concentrations of electron holes and free electrons.
The law of mass action is also useful in the following areas:
- Ecological mathematics
- Sociophysics (social physics)
- Mathematical epidemiology
The mathematical equation of the Law of Mass Action
Consider an imaginary reaction:
A + B → (Products)
As per the Law of Mass Action explanation, the rate of reaction ‘R’ is specified as:
R ∝ A B
For any overall reaction signified by aA + bB → Products, the rate of the reaction conferring to the Law of Mass Action is shown as:
R ∝ Aa Bb
Equilibrium Constant vs. Concentration Quotient
At a particular temperature, the Concentration Quotient (Qc) of a chemical reaction is described as the ratio of the product’s concentrations to the reactant concentrations. The value of Qc will fluctuate as the system reacts, but the Equilibrium concentrations will establish the Equilibrium constant Kc.
If Qc > Kc, the reaction will take place in the opposite direction.
If Qc < Kc, the reaction will take place in the forward direction
If Qc = Kc, the reaction will continue in equilibrium
Other Disciplines’ Laws of Mass Action
Aside from chemistry, the law of mass action relates to a variety of disciplines. Consider the following scenario:
- At equilibrium, the product of electron and hole densities is a constant in semiconductor physics. The Boltzmann constant, temperature, bandgap, and effective density of the valence and conduction band state all influence the constant.
- The diffusion process is related to absolute reaction rates in condensed matter physics.
- The law of mass action is used in sociophysics to describe people’s social and political conduct.
Significance of law of mass action in IIT JEE exam
Because the weightage for the sections is quite low, the chemical kinetics chapter has a weightage of around 3.33 percent in all years. This indicates that the total number of questions from this topic will be about 1 or 2 and will be worth 4 points. The law of mass action and its balancing equation is frequently posed questions in chemical kinetics.
FAQs
Q. When Should the Law of Mass Action Be Used?
Ans: Keep in mind that the law of mass action only holds when the system is in a state of dynamic equilibrium. Make sure the following statements are valid regardless of the arrows in a chemical equation:
- A closed system’s reaction is represented by a chemical equation. In other words, no heat or mass enters or exits the system.
- The temperature does not change. The temperature does not fluctuate when the system is in equilibrium. Similarly, the temperature affects the equilibrium constant of a process. Its value at one temperature could be different from Kc at another.
Q. Write some examples of the law of mass action?
Ans: The law of mass action states that the frequency of any chemical reaction is proportional to the total of the masses of the interacting components, with each mass raised to a power equal to the chemical equation’s coefficient.
Q. What is the active mass representation?
Ans: The molar concentration is usually stated in square brackets as the active mass in units of mol dm3 [.]







