Table of Contents
Acid Rain Ozone and its Reactions: Acid rain also referred to as simply deposition is a general term that relates to any precipitation that contains acid content, like sulfuric or nitric acid, and dropped to the ground in moisture conditions from the atmosphere. This would include acidic rainfall, snow, mist, hail, or perhaps even dust. The pH of most water, even drinking water, is neutral, ranging from 6.5 to 8.5, while acid rain has a pH that is lower, ranging from 4-5 on average. The lesser the pH of acid deposition, the more acid it is. Plants, aquatic animals, and infrastructure can all be harmed by acid rain.
A brief outline
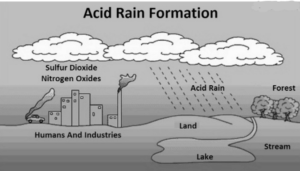
Sulphur dioxide and nitrogen oxide emissions interact with water molecules inside the atmosphere to form acids, resulting in acid rain. Since the 1970s, certain governments have worked to minimize the amount of sulphur dioxide and nitrogen oxide released into the environment. Due to widespread research on acid rain beginning in the 1960s and widely broadcast information on its hazardous consequences, these efforts have had beneficial results. Acid rain is mostly caused by anthropogenic sulphur and nitrogen compounds, but nitrogen oxides can also be formed naturally by lightning strikes, and sulphur dioxide is produced by volcanic eruptions.
Acid rain has been found to have negative effects on forests, freshwaters, and soils, killing bacteria, insects, and aquatic life-forms, peeling paint, corroding steel structures such as bridges, and weathering stone buildings and statues, as well as affecting human health.
Important concepts
Gases that cause acid rain
- Rain that has been acidified, with a pH less than 5.6, is known as acid rain.
- Excess sulphur and nitrogen generated by automobiles and industrial activities interact with rain, resulting in highly acidic precipitation.
- Sulfuric and nitric acids are formed when these pollutants react with water vapours in the atmosphere.
- Particles of sulphur and nitrogen may be released into the atmosphere as a result of manmade or natural processes.
- Industrial emissions, the burning of fossil fuels such as diesel and coal, rubbish incineration, and the creation of paper are all examples of anthropogenic causes.
- Sulfur emitted during volcanic activity or nitrogen ions emitted into the air during a lightning strike could be natural sources. The chemical reaction that produces nitric oxide takes place in the presence of lightning. Nitrogen dioxide is formed as a result of this reaction with oxygen.
- In addition, ozone and other organic acids such as formic and acetic acids contribute 5-20 percent of overall acidity in acid rain.
Acid Rain Effects
- Standing crops and woodlands are harmed by acid rain.
- Freshwater life, other aquatic life forms, insects, and other organisms are all negatively affected.
- Acid rain can lower the pH of the ocean. ‘Ocean acidification is the term for this occurrence. Though acid rain has little effect on the oceans, it has a considerable influence on shallower coastal waterways.
- Increased growth of marine plants and phytoplankton due to excess nitrogen inputs from the atmosphere in the oceans may result in more frequent hazardous algal blooms and eutrophication.
- Because calcium carbonate – the primary component of the limestone skeleton – dissolves in low pH/acidic conditions, the limestone skeleton in corals is sensitive to pH changes.
- Some soil bacteria cannot handle low pH variations and are destroyed as a result. The acid denatures the enzymes of these bacteria.
- It corrodes both constructions and buildings. Acid rain, for example, has turned the marble of the Taj Mahal yellow.
- Acid rain sometimes causes water pipelines to deteriorate, resulting in massive metals like iron, lead, and copper seeping into drinkable water.
- Humans are not instantly harmed by acid rain. Sulfur dioxide causes a variety of health issues. Asthma, bronchitis, and emphysema are all conditions that can be caused by it.
Examples from Real Life
- Acid rain has a serious influence on the Taj Mahal, one of the world’s seven wonders. Many companies in the city of Agra discharge sulphur and nitrogen oxides into the environment. People continue to utilize low-quality coal and firewood as a source of household energy, exacerbating the problem.
- The copper Statue of Liberty, which has been corroded by acid rain and oxidation for over 30 years, is now turning green.
One of the most important stages in minimizing acid rain ozone is to control the anthropogenic origins of acid rain by limiting industrial and automobile emissions. Interventions in policy to limit such emissions are urgently needed. Furthermore, renewable energy sources can help to minimize acid rain by reducing emissions.
Acid Rain Prevention
- The only way to avoid acid rain is to keep a close eye on nitrogen and sulphur emissions.
- Acid rain harms animals, vegetation, and historical sites.
- As responsible citizens, we should be aware of the harm they inflict, as well as the industries that unethically dispose of nitrogen and sulphur compound pollutants.
Also read: NEET Exam Pattern 2022
Frequently Asked Questions
When sulphur dioxide and nitrogen oxides are discharged into the air, a chemical reaction occurs, resulting in acid rain. These substances can ascend to great heights in the sky, where they mix and react to form oxygen and other molecules to produce more acidic pollutants, known as acid rain.
Forests can be severely harmed by acid rain. Acid rain that seeps into the earth can dissolve nutrients that trees require to thrive, such as magnesium and calcium. Aluminium is liberated into the soil as a result of acid rain, making it more difficult for trees to absorb moisture.
The main chemicals that cause acid rain are sulphur dioxide and nitrogen oxide. Because acid is found in fruits, vegetables, and animals, it can have an impact on humans. In other words, if acid rain does not stop and we eat such products, we may become seriously ill. Men are affected by acid rain in general, but not directly. What causes acid rain?
Why is acid rain so dangerous?
What will occur if we don't take action to prevent acid rain?

