Table of Contents
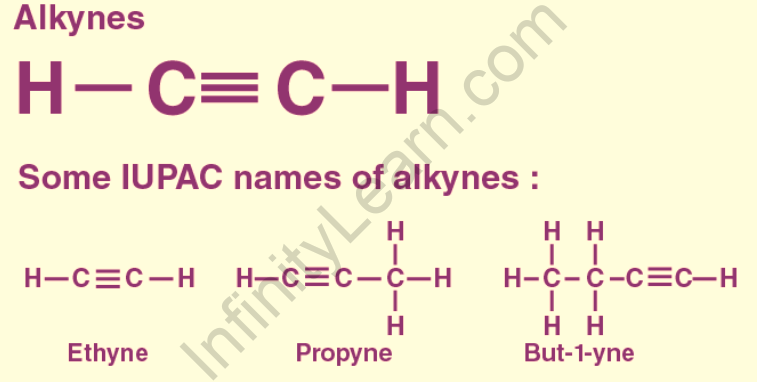
In organic chemistry, an alkyne is an unsaturated hydrocarbon with at least one carbon-carbon triple bond. The simplest acyclic alkynes have a homologous series with the general chemical formula CnH2n-2 and only one triple bond. Alkynes are commonly referred to as acetylenes, though the term acetylene also refers to C2H2, which is formally known as ethyne in IUPAC nomenclature. Alkynes, like other hydrocarbons, are generally hydrophobic. With a bond strength of 839 kJ/mol, the triple bond is extremely strong. The sigma bond contributes 369 kJ/mol of bond strength, the first pi bond contributes 268 kJ/mol of bond strength, and the second pi-bond contributes 202 kJ/mol of bond strength. Bonding is typically discussed in the context of molecular orbital theory, which recognizes the triple bond as the result of s and p orbital overlap. The carbon atoms in an alkyne bond are sp hybridized in the language of valence bond theory: they each have two unhybridized p orbitals and two sp hybrid orbitals. Each atom’s sp orbital overlaps to form one sp–sp sigma bond. Each p orbital on one atom overlaps one on the other, resulting in two pi bonds, for a total of three bonds. Alkynes are named using the Greek prefix system, with no additional letters, in systematic chemical nomenclature. Ethyne and octyne are two examples. It is necessary to specify the location of the triple bond in parent chains with four or more carbons. When the bond begins at the third carbon, octyne can be written as 3-octyne or oct-3-yne. The lowest possible number is assigned to the triple bond. When there are no superior functional groups, the parent chain must include the triple bond even if it is not the longest carbon chain in the molecule. Ethyne is also known as acetylene, which is a more common name for it.
The concept of chemical bonding, combined with quantum mechanics, has revealed a wealth of information about various organic and inorganic compounds that are necessary for life. This article discusses the structure of an organic compound class known as alkynes. Acetylene is the most fundamental member of the alkyne family. Alkynes are unsaturated hydrocarbons with two carbon atoms linked by a carbon-carbon triple bond.
Overview
Alkynes are organic molecules with the empirical formula CnH2n2 that are composed of functional group carbon-carbon triple bonds. They are hydrocarbons that are unsaturated. Alkynes, like alkenes, have the suffix –yne; this suffix is used when there is only one alkyne in the molecule. If a molecule has both a double and a triple bond, the carbon chain is numbered in such a way that the first multiple bonds are assigned a lower number. When both bonds have the same number, the double bond takes precedence. The molecule is then named “n-en-n-yne,” with the double bond root name coming first, followed by the triple bond root name (e.g. 2-hepten-4-yne). The alkynes are unsaturated hydrocarbons with one triple bond; their general formula is CnH2n+2, and the triple bond is known as the ‘acetylenic bond.’ There are numerous alkynes found in nature. With two carbon atoms connected by a triple bond, ethyne (C2H2) is the first member of the alkyne family. An alkyne is an unsaturated hydrocarbon with at least one carbon-carbon triple bond in organic chemistry. Alkynes, like other hydrocarbons, are generally hydrophobic. Ethyne is more commonly known by the innocuous name acetylene.
It is the most basic of the alkynes, with two carbon atoms linked by a triple bond, allowing each carbon to bond to one hydrogen atom.
Unsaturated hydrocarbons are alkenes and alkynes. Alkenes have double bond linkages, whereas alkynes have triple bond linkages.
Propyne
Propyne (methylacetylene) is a type of alkyne with the formula CH3CCH. It is a constituent of MAPD gas, as is its isomer propadiene (allene), which was widely used in gas welding. Propyne, unlike acetylene, can be safely condensed. Propyne is a three-carbon building block that is useful for organic synthesis. Propynyllithium is formed by deprotonation with n-butyllithium. This nucleophilic reagent combines with carbonyl groups to form alcohols and esters. Whereas purified propyne is costly, MAPP gas could be used to generate large amounts of the reagent at a low cost. Propyne, along with 2-butyne, is also used in the total synthesis of vitamin E to synthesize alkylated hydroquinones. An alkynyl proton’s chemical shift and a propargylic proton’s chemical shift usually occur in the same region of the 1H NMR spectrum. These two signals have nearly identical chemical shifts in propyne, resulting in signal overlap, and the 1H NMR spectrum of propyne, when recorded in deuteriochloroform on a 300 MHz instrument, consists of a single signal, a sharp singlet. Propyne is the acetylene family’s second most basic member. It can combine with air and oxidizing agents to form explosive mixtures. It is used to fuel welding torches.
Alkyne examples
A homologous series is a group of carbon compounds that all have the same functional group replacing the hydrogen atom. These compounds have similar chemical properties due to the addition of the same type of functional group in the chain. A homologous series is a collection of hydrocarbons that have the same general formula and chemical properties. They are organic compounds with identical structural and functional groups. The constituents of the homologous series have a range of physical properties.
| Ethyne | C2H2 |
| Propyne | C3H4 |
| 1-Butyne | C4H6 |
| 1-Pentyne | C5H8 |
Also read: Important Topic of Chemistry: Entropy
FAQs
How do alkynes get their names?
Higher alkenes and alkynes are named by appending a -ene (alkene) or -yne (alkyne) suffix to the stem name of the unbranched alkane with that many carbons and counting the number of carbons in the longest continuous chain that includes the double or triple bond.
What exactly is an alkyne group?
Alkynes are organic molecules with the functional group carbon-carbon triple bonds and have the empirical formula CnH2n2. These are unsaturated hydrocarbons. Similar to how alkenes have the suffix –ene, alkynes have the suffix –yne when there is only one alkyne in the molecule.
How do we convert alkyne to alkene?
Alkynes can be converted to trans-alkenes by dissolving sodium in ammonia. A Na radical donates an electron to one of the P bonds in a carbon-carbon triple bond. This produces an anion in an ammonia solution, which can be protonated by hydrogen.



