Table of Contents
Fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (A) is a set of five or six chemically related elements in the periodic table (At). Tennessine (Ts), a man-made element, has the potential to be a halogen. In modern IUPAC nomenclature, this group is designated as group 17. “Halogen” is a word that means “salt former.” When halogens react with metals, a variety of salts are formed, including calcium fluoride, sodium chloride (common table salt), silver bromide, and potassium iodide. At standard temperature and pressure, the halogen group is the only periodic table group that contains elements in three of the four main states of matter. When halogens are bonded to hydrogen, they all react to form acids. The majority of halogens are derived from minerals or salts. As disinfectants, the middle halogens—chlorine, bromine, and iodine—are frequently used. The most common type of flame retardant is organobromides, whereas elemental halogens are hazardous and can be toxic. The halogens fluorine, chlorine, bromine, and iodine are nonmetals, and the chemical properties of the two heaviest groups 17 members have yet to be determined. The halogens’ chemical bond energy goes down the periodic table column from top to bottom, with fluorine deviating slightly. It has the highest bond energy in compounds with other atoms, but it has extremely weak bonds within the diatomic F2 molecule. This means that the reactivity of elements decreases as one moves down group 17 in the periodic table due to the increasing size of the atoms.
All halogens combine with hydrogen to form binary compounds known as hydrogen halides, which include hydrogen fluoride (HF), hydrogen chloride (HCl), hydrogen bromide (HBr), hydrogen iodide (HI), and hydrogen astatide (HAt). When these compounds are combined with water, they all react to form acids. The only hydrogen halide that forms hydrogen bonds is hydrogen fluoride. Strong acids include hydrochloric acid, hydrobromic acid, hydroiodic acid, and hydrostatic acid, but hydrofluoric acid is a weak acid.
Overview
On the periodic table, halogens are a group of elements. It is the only element group that includes elements capable of existing at room temperature in three of the four main states of matter: solid, liquid, and gas. Because halogens react with metals to produce many important salts, the word halogen means “salt-producing.” In fact, halogens are so reactive that they do not exist in nature as free elements. Many, on the other hand, are common in combination with other elements. Here’s a look at what these elements are, where they are on the periodic table, and what they have in common.
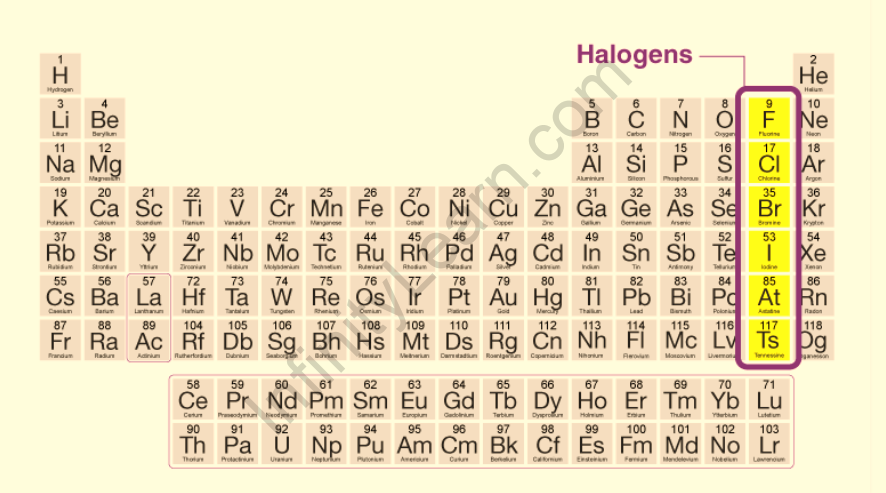
The halogens are found in Periodic Table Group VIIA, or Group 17 in IUPAC nomenclature. The element group is a subset of nonmetals. They are located in a vertical line on the right side of the table. Nonmetals are halogens. Fluorine and chlorine are gases at room temperature, while bromine is a liquid. Solids are iodine and astatine. Halogens are extremely reactive, with decreasing reactivity from fluorine to astatine. In nature, halogens do not exist in their elemental form. Astatine isotopes have short half-lives and are radioactive.
What do table salt, bleach, fluoride in toothpaste, and chlorine in swimming pools have in common? When halogen lamps are added to the list, the answer becomes clearer: they all involve one or more of the halogens, which comprise Group 7 of the periodic table, which consists of five chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (A) (At). The term “halogen” is derived from Greek and means “salt-forming.” Because of their high reactivity, halogens are only found in the environment as ions or compounds.
Halogen group
The halogen group, or Group 17 of the periodic table, consists of five elements that play important roles in our daily lives, such as fluorine in toothpaste and chlorine as a disinfectant to clean water.4
Group 17’s five members are as follows:
Fluorine
Chlorine
Bromine
lodine
Astatine
These elements are referred to collectively as halogens. The term halogen is derived from the Greek words halo, which means salt, and genes, which means born. As a result, halogens in Greek refer to salt producers. Despite being a radioactive element, astatine is classified as a halogen because its behaviour is similar to that of iodine.
Halogen elements
There are either 5 or 6 halogens, depending on who you ask. Halogens include fluorine, chlorine, bromine, iodine, and astatine. Tennessine, element 117, may share some properties with the other elements. Despite being in the same periodic table column or group as the other halogens, most scientists believe element 117 behaves more like a metalloid. Because so little of it has been produced, it is a matter of prediction rather than empirical data.
Fluorine
Chlorine
Bromine
Iodine
Astatine
Tennessine (might behave as a halogen, at least in some respects)
Use of halogens
Because of their high reactivity, halogens are excellent disinfectants. Two well-known examples are chlorine bleach and iodine tincture. Organobromine compounds, also known as organobromides, are used as flame retardants. Salts are formed when halogens react with metals. The chlorine ion, which is typically obtained from table salt (NaCl), is required for human survival. Fluoride is a type of fluoride that is used to help prevent tooth decay. Halogens are also used in lighting and refrigerants.
The lighter halogens can be found in living organisms. Fluorine, chlorine, bromine, and iodine are examples of these elements. Chlorine and iodine are essential for human nutrition, though the other elements may be required in trace amounts as well.
Halogens play an important role in disinfection. Water and surfaces are disinfected using chlorine and bromine. Because of their high reactivity, these elements are also important components of some types of bleach. Incandescent lamps contain halogens, which cause them to glow at a higher temperature and in white colour. The halogen elements are important to drug components because they help drugs penetrate tissues.
Halides with less electronegative elements are formed by halogens. Metal halides range from ionic to covalent, whereas nonmetal halides are covalent. Interhalogens are formed by combining two or more different halogens. Minerals are directly reacted with all of the representative halogen elements or with hydrohalic acid solutions (HF, HCl, HBr, and HI) to produce representative metal halides. In the addition of aqueous hydrohalic acids, basic anions such as hydroxides, oxides, or carbonates are involved.
FAQs
What are the Overarching Trends in Halogen Chemistry?
There are several patterns in halogen chemistry that do not require double or triple bonds to explain the chemistry of halogens. Fluorine chemistry has been simplified by the fact that it is the first electronegative element in the tabular array and that it has no d orbitals in its valence shell, so it cannot expand its valence shell. Chlorine, bromine, and iodine all have valence shell d orbitals that can be extended to carry up to 14 valence electrons. The chemistry of the halogens is conquered by oxidation-reduction reactions.
Q. What are the applications of halogen?
Ans: The following are some of the most common applications for halogens:
- Fluorine compounds are commonly found in toothpaste and drinking water supplies. Fluorine is a very useful medicine because it is highly reactive with tooth enamel and delays tooth decay.
- Chlorine is widely used in the bleaching process. It is also used in the metallurgy of gold and platinum.
- Chlorine is used in the purification of drinking water.
- Iodine is an antiseptic and a good germicide.



