Table of Contents
Sulfur is a chemical substance with the atomic number 16 and the symbol S. It’s nonmetallic, plentiful, and multivalent. Sulfur atoms form cyclic octatomic molecules with the chemical formula S8 under normal circumstances. At ambient temperature, element sulfur is a bright yellow crystalline solid. Sulfur is the tenth most plentiful element in the universe and the fifth most prevalent on Earth in terms of mass. Sulfur on Earth is mostly found in sulfide and sulfate minerals, while it is occasionally found in its pure, native form. The manufacturing of sulfuric acid for sulfate and phosphate fertilizers, as well as other chemical processes, is the element’s most common commercial application.
A Brief Outline
Sulfur is required for all living things; however, it is nearly always found in the form of compounds or metal sulfides. Organosulfur compounds include three amino acids (cysteine, cystine, and methionine) and 2 vitamins (biotin and thiamine). The phase transition has little effect on the structure of the S8 ring, but it does influence the intermolecular interactions. Between melting and boiling temperatures, octa sulfur changes allotropes again, switching from -octa sulfur to -sulfur, with a decreased density but increased viscosity due to polymer formation. As depolymerization happens at higher temperatures, the viscosity falls.
Sulfate is largely produced from the environment in most forest ecosystems, with some sulfur contributed through weathering of ore minerals and evaporites. The isotopic composition of sulfur has been used to detect pollution sources, and enriched sulfur has been utilized as a tracer in hydrologic research.
Important Concepts
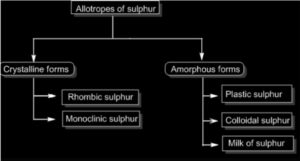
Allotropic forms
Sulfur in a variety of allotropic forms that are all in the same physical state However, rhombic or octahedral (– sulfur) and monoclinic sulfur (– sulfur) is the most important crystalline forms. At temperatures below 96oC, we find the shape of rhombic sulfur. Monoclinic sulfur, on the other hand, forms at temperatures above 96°C.
The changeover temperature in between two crystalline formations is 96 degrees Celsius. Polymeric sulfur is yet another allotrope of sulfur (S8). It’s a ring particle with eight parts. Organic media synthetic and natural rubber are all insoluble in this. It’s also not soluble in carbon disulfide.
Allotropic forms of Sulphur
1) Rhombic Sulphur
- They appear as yellow, translucent crystals.
- The melting point of the Rhombic Sulphur is 114o
- Sulphur rhombic has a density of 2.08 g/cm3.
- It remains stable at temperatures below 96 degrees Celsius.
2) Monoclinic Sulphur
- These crystals are clear and amber in color.
- They have a melting point of 119 degrees Celsius.
- Monoclinic sulfur has a density of 1.98 gcm3.
- At temperatures below 96°C, it becomes unstable and takes on a rhombic shape.
- It’s important to know that rhombic sulfur transforms into kaleidoscopic or prismatic sulfur at temperatures of 96°C or above. At 96 degrees Celsius or lower, kaleidoscopic or prismatic sulfur transforms into rhombic sulfur.
- Enantiotropic Allotropes are allotropes that shift their configuration from one form to another as the temperature changes.
3) Colloidal Sulphur
- This sulfur can be made by passing hydrogen sulfide across a saturated and cooled sulfur dioxide solution in water. Another option is to add an alcohol and sulfur solution to the water.
- In carbon disulfide, it works as a solvent.
- We use it as a component of pharmaceuticals.
4) Milk Sulphur
- We can make this by using mild hydrochloric acid to react with ammonium sulfide. Sulfur milk is produced in a similar way by heating sulfur with calcium hydroxide (aqueous solution). To obtain sulfur-free milk, we filter this combination and add mild hydrochloric acid.
- This substance has a non-crystalline structure and is white in color.
- Carbon di-sulfide dissolves it.
5) Plastic Sulphur
- When molten Sulphur is thrown into cold water, it forms gamma–Sulphur, which is a stiff, elastic solid. It’s a liquid that’s been supercooled.
- Sulfur is an octahedral ring in its yellow powdered form. This ring is broken apart by heat. When molten Sulphur is cooled by immersing it in water, it forms a sequence of covalent links or chains, similar to a pliable carbon polymer.
Most stable allotropic form of Sulphur
The yellow rhombic allotrope of sulfur, commonly known as -sulfur, is the most stable and consistent allotrope of sulfur. When the rhombic structure is heated to temperatures more than 370 K, the rhombic structure transforms into monoclinic, also known as -sulfur. Octahedral sulfur is another name for rhombic sulfur. Rhombic sulfur is immiscible in water but perfectly miscible in organic solvents like benzene, ether, and others like alcohol.
Sulfur is used in a variety of ways.
- Sulphuric acid, sulfur dioxide, and other sulfur compounds are all made from it.
- Sulpha medicines and ointments for a skin condition are made in medicine.
- Rubber is vulcanized using this method.
- It’s used to make black gun powder, a blend of carbon (charcoal), sulfur, and nitrate (KNO3).
- It’s utilized in the production of pesticides and fungicides.
Significance of compounds of Sulphur in NEET exam
To expert the NEET test, you should get a handle on your themes’ major ideas in general. For this, answers should be enormously improved and introduced in an intelligible way, utilizing somewhat straightforward methodology and fewer estimations. This permits you to save time and exertion during the test. For web-based learning and comprehension of ideas, live classes are accessible. Basic free pdfs are likewise accessible in disconnected mode. Subsequently, learning and taking notes occur simultaneously. By getting to these free PDFs, you can get a total arrangement of notes.
FAQ’s
Because of its applications, sulfur has taken on a new significance. Its applications are not restricted to the industrial sector; it also plays an important function in our ecology by influencing plant growth. As a result, numerous sulfur-containing fertilizers have been developed.
The impact of sulfur has increased as a result of its applications. Its uses aren't limited to industry; it also has a significant impact on our environment by influencing plant development. As a result, a number of sulfur-based fertilizers have been created.
The quenched result of sulfur that melts over 160°C is known as amorphous sulfur (at this temperature, the liquid characteristics melt and alter dramatically). For instance, a significant increase in viscosity). Its appearance gradually shifts from a plastic to a glassy state. Glassy, vitreous, or plastic sulfur are some of its other names. -sulfur is another name for it. It contains a complicated blend of catena-sulfur that is combined with cyclo-forms. What is the significance of sulfur?
What is the impact of sulfur?
What is amorphous sulfur, and what is it used for?





