Table of Contents
Introduction
An electrochemical cell is a device that can generate electrical energy from chemical reactions or use electrical energy to initiate chemical reactions. Electrochemical cells that generate an electric current are known as voltaic or galvanic cells, whereas those that generate chemical reactions, such as electrolysis, are electrolytic cells. A standard 1.5 volt cell intended for consumer use is a common example of a galvanic cell. A battery is made up of one or more cells that are connected in parallel, series, or series-and-parallel fashion.
In chemistry, a cell is used to convert chemical energy to electrical energy, or for the purpose of energy conversion. Chemical reactions take place inside the cells, and as a result, electrical current flows. In this article, we will go over two types of cells in-depth: galvanic cells and electrolytic cells. In galvanic cells, chemical energy is converted into electrical energy, and electrical energy is converted back into chemical energy in electrolytic cells. These electrochemical cells are used for a variety of purposes.
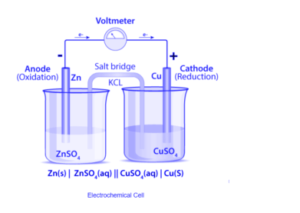
Galvanic cells and Voltaic cells are examples of cells that can generate an electric current as a result of chemical reactions. Electrolytic cells, on the other hand, are the cells that cause chemical reactions when an electric current is passed through them. Electrochemical cells are made up of two half-cells, each of which contains an electrode dipped in an electrolyte. Both half cells can use the same electrolyte. These half cells are linked by a salt bridge, which serves as a platform for ionic contact between them without allowing them to mix. A salt bridge is a piece of filter paper that has been dipped in a potassium nitrate or sodium chloride solution.
Overview
A dry cell is a type of electric battery that is commonly used in household and portable electronic devices. A battery is a device that converts chemical energy into electrical energy by using one or more electrochemical cells. A dry cell is one of the electrochemical cells developed by “German scientists Carl Gassner” in 1886, following Georges Leclanche’s development of wet zinc-carbon batteries in 1866. Yai Sakizo, a Japanese inventor, invented modern dry cells in the year 1887. Dry cell batteries, which range from large flashlight batteries to small flashlight batteries and are mostly used in wristwatches or calculators, are the most commonly used batteries today. A dry cell is an electrochemical cell made up of low moisture immobilized electrolytes in the form of a paste that prevents the electrolytes from flowing. As a result, it is easily transportable.
A dry cell is a device that uses chemical reactions to generate electricity. When the cell’s two electrodes are connected by a closed path, the cell forces electrons to flow from one end to the other. In a closed circuit, the flow of electrons causes current to flow. Electrons flow from one end to the other using chemical reactions.
Because of the high potential, more electrons flow when two or more cells are connected with the correct polarity. This is referred to as a battery. A battery can be used to generate a range of voltages ranging from 1.5 V to 100 V. Using power electronic converters such as chopper circuits, the output DC voltage of the battery can also be regulated to different levels.
Dry cell
A dry cell is primarily based on chemical reactions. Electrons flow from one electrode to the other as a result of reactions between the electrolyte and the electrodes.
Acids, for example, dissolve in water to form ionized particles. There are two kinds of ionized particles. The positive ions are known as cations, while the negative ions are known as anions. Electrolytes are acids that are dissolved in water. The electrolyte in the above-mentioned diagram is zinc chloride. Ammonium chloride jelly, on the other hand, forms as an electrolyte. Electrodes are formed by metal rods immersed in electrolytes. We have a positive electrode as the anode and a negative electrode as the cathode based on the chemical properties of the metal rods.
The oppositely charged ions are drawn to the electrodes. The cathode, for example, attracts anions while the anode attracts cations. Electrons flow from one direction to the other in this process, resulting in a charge flow. This is referred to as current. The dry cell can be classified as a primary cell or a secondary cell depending on its nature. A primary cell is one that cannot be reused or recharged. When all of the chemical reagents are consumed, the electrochemical reactions fail to produce electricity. A secondary cell, on the other hand, can be recharged by using battery charges to regenerate the chemical reactions.
The chemical reactions between the electrode and the electrolytes are at the heart of the dry cell’s operation. When the electrodes are immersed in electrolytes, they attract oppositely charged ions to themselves. This results in the flow of charges and, as a result, the generation of current.
Galvanic cell
A galvanic cell is an electrochemical cell in which spontaneous redox processes occur, allowing the continuous flow of electrons through the conductor, whereas in an electrolytic cell, redox reactions are induced by an external source of current. In a galvanic cell, mechanical energy is converted to electrical energy, and electrical energy is converted to chemical energy in an electrolyte cell. A galvanic cell or voltaic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. A voltaic cell is an electrochemical cell that generates electrical energy through chemical reactions.
Electrons are transferred from one species to another in oxidation-reduction reactions. If the reaction occurs spontaneously, energy is released. As a result, the released energy is put to good use. To deal with this energy, the reaction must be split into two separate half-reactions, namely oxidation, and reduction. The reactions are put into two different containers and wires to drive the electrons from one end to the other. This results in the formation of a voltaic cell.
The Gibbs energy of spontaneous redox reaction in the voltaic cell is primarily responsible for the electric work done by a galvanic cell. It is typically made up of two half cells and a salt bridge. Each half cell also includes a metallic electrode dipped in an electrolyte. With the help of metallic wires, these two half-cells are externally connected to a voltmeter and a switch. When both electrodes are dipped in the same electrolyte, a salt bridge is not always required.
When an electrode is exposed to the electrolyte at the electrode-electrolyte interface in a galvanic cell, the atoms of the metal electrode tend to generate ions in the electrolyte solution, leaving the electrons at the electrode behind. As a result, the metal electrode becomes negatively charged. Metal ions in the electrolyte solution, on the other hand, have a tendency to deposit on a metal electrode. As a result, the electrode becomes positively charged. Charge separation is observed under equilibrium conditions, and the electrode can be positively or negatively charged depending on the tendencies of two opposing reactions. As a result, a potential difference develops between the electrode and the electrolyte. This difference in potential is referred to as electrode potential. The electrode that undergoes oxidation is known as the anode, while the electrode that undergoes reduction is known as the cathode. The anode has a negative potential toward the solution, whereas the cathode has a positive potential toward the solution. As a result, a potential difference develops between the galvanic cell’s two electrodes. This difference in potential is referred to as cell potential. When no current is drawn from the galvanic cell, the electromotive force of the galvanic cell is known as cell potential. When the switch is turned on, electrons flow from the negative electrode to the positive electrode due to the potential difference.
Galvanic cell example
More than a century ago, electrochemical or galvanic cells were introduced as a tool for studying the thermodynamic properties of fused salts. Daniel’s cell is a galvanic cell that converts chemical energy to electrical energy. Copper ions are reduced at the cathode and zinc ions are oxidized at the anode in Daniel’s cell.
Daniel cell reactions at the cathode and anode are as follows:
At cathode: Cu 2+ + 2e– → Cu
At anode: Zn → Zn2+ + 2e–
Electrolysis cell
An electrolytic cell is an electrochemical device that uses electrical energy to promote a non-spontaneous redox reaction. Electrolytic cells are electrochemical cells that can be used to electrolyze specific compounds. Water, for example, can be electrolyzed (using an electrolytic cell) to produce gaseous oxygen and gaseous hydrogen. This is accomplished by utilizing the flow of electrons (into the reaction environment) to overcome the non-spontaneous redox reaction’s activation energy barrier.
The following are the three primary components of electrolytic cells:
- The cathode (which is negatively charged for electrolytic cells)
- The anode (which is positively charged for electrolytic cells)
- Electrolyte
The electrolyte serves as a medium for electron exchange between the cathode and the anode. Water (containing dissolved ions) and molten sodium chloride are two common electrolytes used in electrolytic cells.
With the help of an electrolytic cell, molten sodium chloride (NaCl) can be electrolyzed, as shown below. In this experiment, two inert electrodes are immersed in molten sodium chloride (which contains dissociated Na+ cations and Cl– anions). When an electric current is passed through the circuit, the cathode becomes electron-rich and acquires a negative charge. Positively charged sodium cations are now drawn to the negatively charged cathode. As a result, metallic sodium is formed at the cathode. At the same time, chlorine atoms are drawn to the positively charged cathode. As a result, chlorine gas (Cl2) is produced at the anode (which is accompanied by the liberation of 2 electrons, finishing the circuit).
FAQ’s
What are the primary distinctions between electrolytic and galvanic cells?
Electrolytic cells' cell reactions are non-spontaneous, whereas Galvanic cells' cell reactions are spontaneous. Galvanic cells produce electrical energy from chemical reactions, whereas electrolytic cells produce non-spontaneous redox reactions in response to electrical energy input.
What are the three most important components of electrolytic cells?
The cathode, anode, and electrolyte are the three main components of electrolytic cells. In electrolytic cells (as in most electrochemical cells), oxidation takes place at the anode and reduction takes place at the cathode.
What Are the Primary Distinctions Between Cathode and Anode?
The cathode of an electrochemical cell is where reduction takes place. A positive (+) sign is commonly used to represent it. Electrons move from the anode to the cathode. The anode is the electrode in electrochemical cells where oxidation takes place. It is denoted by a minus (-) sign.





