Table of Contents
Effects of depletion of the ozone layer: Did you know that depletion of ozone layer is one of the growing concerns now? What is ozone layer and what happens when it depletes? In this article, we will get all the information on ozone layer and the effects of depletion.
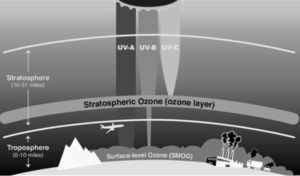
The ozone layer, also characterized as the ozone barrier, is an area of the Stratosphere that traps the majority of UV radiation from the Sun. It has a significant ozone (O3) concentration in contrast to other areas of the atmosphere, although it is still small in comparison to other gasses in the stratospheric.
- The ozone layer has a concentration of fewer than 10 parts per million, while the typical ozone concentration in the entire atmosphere is around 0.3 parts per million.
- Although its thickness varies seasonally and geographically, the ozone layer is primarily located in the lower stratosphere, between 15 and 35 kilometres above Earth.
A brief outline about Effects of depletion of the ozone layer
- Sydney Chapman, a British physicist, identified the photochemical principles that cause the ozone layer to form in 1930. Ultraviolet radiation strikes regular oxygen molecules containing two oxygen atoms (O2) in the Earth’s stratosphere, shattering them into individual oxygen atoms (atomic oxygen); the atomic oxygen subsequently mixes with unbroken O2 to form ozone, O3.
- The ozone molecule is unstable (though long-lived in the stratosphere), and when UV light strikes it, it splits into a molecule of O2 and a single atom of oxygen, a process known as the ozone-oxygen cycle. Despite the fact that ozone concentration in the ozone layer is very low, it is necessary to live because it absorbs potentially harmful ultraviolet (UV) light from the sun.
- Because most UV-A is absorbed by ozone, the majority of this longer-wavelength UV radiation reaches the surface, accounting for the majority of UV reaching the Earth. Although this type of UV radiation is less destructive to DNA, it nevertheless has the potential to cause physical harm, premature skin ageing, indirect genetic damage, and skin cancer.
Important concepts:
Depletion of the Ozone layer
- The bringing down of the ozone layer in the high climate is alluded to as ozone layer exhaustion. At the point when chlorine and bromine iotas in the air come into contact with ozone, the ozone particles are obliterated.
- One particle of chlorine can obliterate 100,000 ozone atoms. It is drained quicker than it is made. At the point when certain synthetic compounds are presented to extreme bright radiation, they emanate chlorine and bromine, which adds to the ozone layer’s consumption. Ozone Depleting Substances will be substances that drain the ozone layer (ODS).
- Chlorofluorocarbons, carbon tetrachloride, hydrochlorofluorocarbons, and methyl chloroform are all ozone-draining synthetics that contain chlorine. Halons, methyl bromide and hydro Bromo fluorocarbons are ozone-exhausting synthetic compounds that incorporate bromine.
- The most common ozone-depleting chemical is chlorofluorocarbons. The only time the chlorine atom does not react with ozone is when it reacts with another molecule.
- In 1987, the Montreal Protocol was proposed to prohibit the use, manufacturing, and import of ozone-depleting compounds, as well as to reduce their concentration in the atmosphere, in order to safeguard the earth’s ozone layer.
Ozone Layer Depletion Causes
Depletion of the ozone layer is a major worry that is linked to a variety of factors. The following are the primary factors that contribute to the ozone layer’s depletion:
- Chlorofluorocarbons
The main source of ozone layer depletion is chlorofluorocarbons or CFCs. Solvents, spray aerosols, freezers, and air conditioners, among other things, emit these. The ultraviolet radiations in the stratosphere break down chlorofluorocarbon molecules, releasing chlorine atoms. These atoms degrade ozone by reacting with it.
- Rocket Launches That Aren’t Regulated
According to studies, the unregulated launch of rockets depletes the ozone layer far more than CFCs do. If not addressed, this might result in a significant depletion of the ozone layer by 2050.
- Compounds containing nitrogen
The loss of the ozone layer is mostly caused by nitrogenous chemicals such as NO2, NO, and N2O.
- Natural Factors
Certain natural phenomena, such as Sunspots and stratospheric winds, have been discovered to degrade the ozone layer. However, it only contributes to a 1-2 percent reduction in ozone layer depletion. Volcanic eruptions are also to blame for the ozone layer’s depletion.
- Ozone Layer Depletion’s Consequences
The ozone layer’s depletion has negative consequences for the environment. Let’s look at the key consequences of ozone depletion on humans and the environment.
- Human Health Effects
Due to the ozone layer’s depletion, humanity will be directly exposed to the sun’s dangerous UV radiation. Humans may have major health problems as a result, including skin illnesses, cancer, sunburns, cataracts, rapid ageing, and a weakened immune system.
- Animal Reactions
In animals, direct exposure to UV light causes skin and eye cancer.
Because most energetic UV light cannot reach the Earth’s surface due to ozone in the atmosphere, astronomical data in these wavelengths must be acquired from satellites orbiting above the atmosphere and ozone layer. Because most of the light from newborn hot stars is in the ultraviolet, researching these wavelengths is crucial for understanding galaxies’ origins.
GALEX is an orbiting ultraviolet space telescope that was launched on April 28, 2003, and was operational until early 2012.
Also Read: Ozone Layer Depletion, Meaning, Facts, Causes, Effects, Prevention, and Solutions
FAQs on Effects of depletion of the ozone layer
Plants might have little development, blossoming, and photosynthesis because of solid bright light. The forests are likewise exposed to the adverse effects of UV radiation.
Openness to harming UV radiation essentially affects tiny fishes. These creatures are at the head of the amphibian order of things. The animals in the pecking order are additionally hurt on the off chance that the tiny fishes are killed.
Due to a variety of human activities, the world is becoming warmer. Pollutants from industrial processes, such as factory smoke, as well as home and residential burning of chemicals, are included. Another cause is the use of non-renewable resources such as fossil fuels. The smoke released by such sources contributes to climate change and instability. As a result, the world is experiencing unpredictable climatic fluctuations and natural disasters. The unsustainable use of these resources and the disregard for environmental concerns will cost a lot of money. What are the environmental effects due to ozone depletion?
What are the marine effects due to ozone depletion?
What is the definition of global warming?

