Table of Contents
Introduction
The smallest unit of a chemical substance is referred to as a molecule. It has the same chemical properties as the compound in concern. Because molecules are made up of atoms that are linked together by chemical bonds, there is a lot of room for size and complexity variation. In chemistry, the molecule is said to be the smallest unit of a substance that holds the compound’s chemical properties. They are built up of atoms arranged in groups. When explaining an atom’s structure, it is also fragmented into smaller components. Protons, electrons, and neutrons are the sub-particles of an atom. The nucleus of the atom, which is surrounded by electrons, contains protons and neutrons. We can say that both the empirical and molecular formula can be useful depending on the circumstances. In most cases, the empirical formula is employed to simply demonstrate which elements are present in a molecule. This is useful when you want to know what elements you’re dealing with at a glance.
The information about empirical and molecular formulas from various physics-related articles is available here. Empirical formulas and molecular formulas are important topics in physics. Students who want to flourish in physics need to be well known about this to get deep knowledge about it to do well on their exams. The definitions, brief explanations, and differences are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional physics help.
Overview
When you want to know how many atoms of each element are present in a compound, the molecular formula comes in handy. It provides more information than the empirical formula and is thus more widely used. When you begin working with organic chemistry, the molecular formula becomes very significant.
When working with molecular and empirical formulas, the most important thing to remember is to divide the subscripts of the molecular formula to produce the empirical formula.
Combustion analysis is used to determine a compound’s empirical formula but not its molecular formula (other techniques can though). Once the empirical formula is known, the molecular formula can be calculated.
Empirical Formula Definition
The chemical formula that is the simplest form of the molecular formula is the empirical formula for molecules. This can be determined by dividing all of the formula’s subscripts by their LCD (Lowest Common Denominator). When a formula is simplified, it becomes an empirical formula. The molecular formula, which is a multiple of the empirical formula, is often employed.
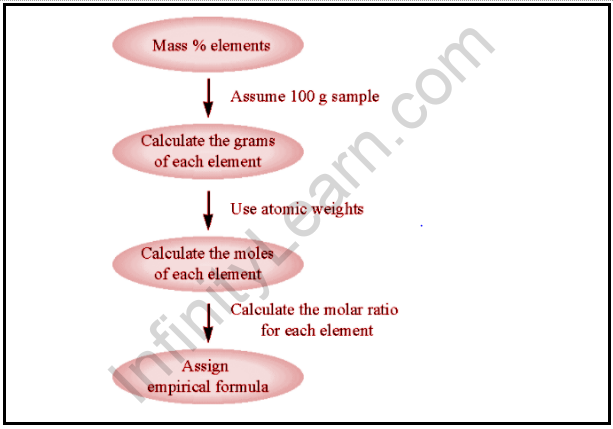
Steps for Empirical Formula Calculation:
- Start with the number of grams of each ingredient that you typically find in an experiment or have provided in a challenge.
- Assume the total mass of a sample is 100 grams to simplify the computation and allow you to work with basic percentages. In other words, make each element’s mass equal to the percentage. The sum should be one hundred percent.
- To convert the mass of each element into moles, multiply the atomic weight of the elements in the periodic table by their molar mass.
- Divide each mole value by the small number of moles you calculated.
- You should round each number to the next whole number. The full numbers represent the mole ratio of the elements in the compound, which are represented by the subscript numbers that appear after the element symbol in the chemical formula.
Molecular Formula Definition
The molecular formula would be the chemical formula for molecules that includes the integer amount of each atom that you calculated or were given. When formulating a formula, the integer amounts of the atoms are included in the subscript. Molecular formulas are assigned gram molecular masses that are simple whole number multiples of the empirical formula mass.
Molecular Formula = n × Empirical Formula
The molar mass of the chemical must be known in order to determine its molecular formula. To estimate the molar mass of compounds, chemists utilize a device known as a mass spectrometer.
The steps to get the molecular formula:
- First, divide the compound’s molar mass by the empirical formula molar mass.
- The outcome should be a full number or extremely close to one.
- Multiply the whole number found in the previous step by all the subscripts in the empirical formula. Then, we will get the molecular formula.
Empirical Formula and Molecular Formula
The empirical formula is always the chemical formula for a compound found by composition analysis. If we know the molecular weight of the compound, we can deduce the chemical formula from the empirical formula. The chemical formula will always be a multiple of the empirical formula in some integer (i.e. integer multiples of the subscripts of the empirical formula).
Combustion Analysis
If a carbon-hydrogen complex is burned with oxygen in a particular combustion device, all of the carbon is transformed to CO2 and all of the hydrogens is converted to H2O. The amount of CO2 created can be used to calculate the amount of carbon produced. This is caught by the sodium hydroxide, and we can track the amount of CO2 created by measuring the rise in the mass of the CO2 trap. Similarly, the quantity of H generated by the amount of H2O trapped by the magnesium perchlorate can be calculated.
Difference between Empirical and Molecular Formula
The empirical formula differs from the molecular formula in that the empirical formula indicates the simplest ratio of atoms involved in the compound, whereas the empirical molecular formula quantifies the number of atoms of an element present in the compound. An empirical formula shows the proportion of a compound’s elements.
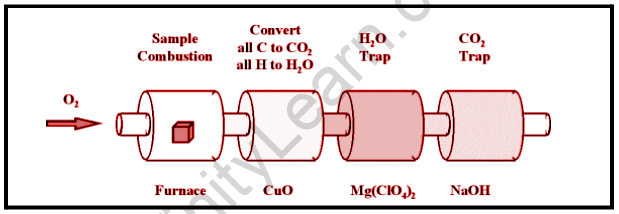
An analytical process known as combustion analysis is one of the most frequent approaches to evaluate the elemental makeup of an unknown hydrocarbon. A small, precisely weighed sample of an unknown molecule containing carbon, hydrogen, nitrogen, and/or sulfur is burned in an oxygen atmosphere. Alternative elements, such as metals, can be identified using other procedures. and the amounts of the resultant gaseous products are determined using one of several ways.
Also read: Concept of Elements, Atoms, and Molecules
FAQs
What is the molecular formula?
The molecular formula would be the chemical formula for molecules that includes the integer amount of each atom that you calculated or were given. Molecular formulas are assigned gram molecular masses that are simple whole number multiples of the empirical formula mass.
What is the empirical formula?
The chemical formula that is the simplest form of the molecular formula is the empirical formula for molecules. This can be determined by dividing all of the formula's subscripts by their LCD (Lowest Common Denominator). When a formula is simplified, it becomes an empirical formula.
How do you find the molecular formula from an empirical formula?
The chemical or molecular formula will always be a multiple of the empirical formula in some integer (i.e. integer multiples of the subscripts of the empirical formula).





