Table of Contents
When an acid and a base are mixed, they undergo a neutralization reaction, as we saw in the section on chemical reactions. The term “neutralization” appears to indicate that a stoichiometrically equal acid-base solution is neutral. While this is occasionally true, the salts generated in these reactions may have their own acidic or basic properties. When the concentrations of hydronium and hydroxide ions in a solution are equal, it is called neutral. An acid-base neutralization reaction happens when acid and base solutions are mixed. Even if we combine stoichiometrically identical quantities, the resulting solution may not be neutral. Because the composition of the salt generated affects whether the solution is acidic, neutral, or basic, it could contain either an excess of hydronium ions or an excess of hydroxide ions.
The information about salt hydrolysis from various chemistry-related articles is available here. It is observed that hydrolysis of salts refers to the process of salt ions interacting with water ions. Students who want to flourish in chemistry need to be well known about salt hydrolysis to get deep knowledge about it to do well on their exams. The definition and brief explanations are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional chemistry help.
Overview
The presence of hydronium ions gives Bronsted-Lowry acids their distinctive properties in aqueous solutions; those of Bronsted-Lowry acids in aqueous solutions don’t. -The presence of hydroxide ions causes lowry bases. The reaction of the hydronium and hydroxide ions to generate water causes the neutralization that occurs when aqueous solutions of acids and bases are mixed. Because of the salts generated during neutralization reactions, the resultant solutions may be somewhat acidic or basic. The extent of the hydrolysis of the ions in the solution determines the pH of solutions containing salts or hydrated metal ions.
When an acid and a base react, they generate a salt, which is an ionic substance. Salt solutions are typically acidic or basic, despite the fact that they appear to be neutral. That is, because of competing hydrolysis processes between the cation and the anion, salts generated by the interaction of a weak acid and a weak base are more difficult to study. The cations or anions formed during the ionization of salts might exist as hydrated ions in aqueous solutions or interact with water to regenerate acids and bases. Salt hydrolysis is the process of salt cations or anions interacting with water.
Hydrolysis of Salts
The chemistry of salt hydrolysis involves the production of salt as the consequence of the neutralization reaction between an acid and a base. In water, most salts ionize to generate acids and bases. These salts dissolve into their individual ions, which can exist as hydrated ions in aqueous solutions or interact with the solvent’s hydrogen or hydroxyl ions to generate bases and acids. The term “hydrolysis of salts” refers to the process of salt ions interacting with water ions.
Salts are classified as follows based on the definition of salt hydrolysis and the degree of hydrolysis:
- Basic salt
- Salt
- Neutral or amphoteric salts.
The kind of salt hydrolysis determines the creation of these salts. They are as follows:
Salts of a Strong Base and a Strong acid:
In nature, salts formed by the interaction of a strong base and a strong acid are neutral. The bonds produced between the anion and the cation are extremely strong and do not dissolve in solution. Following the establishment of the link between them, the electronic distribution of both the cation and the anion is such that both ions have reached their most stable electronic state. Ionic or electrostatic bonding generates these salts, which cannot be broken in solution. Although these salts are not hydrolyzed, they can be hydrated. These salts are known as neutral or amphoteric salts because they have no charge. A famous example of a neutral salt is sodium chloride (NaCl).
Salts of a Weak Acid and a Strong Base:
Positively charged salts are generated when a weak acid and a strong base are neutralized. A basic salt is what these salts are termed. These salts are easily hydrolyzed in water. Sodium acetate, for example. Sodium acetate’s salt hydrolysis formula is as follows:
CH3COONa(aq)→CH3COO–(aq)+Na+(aq)
The generated acetate ion reacts with the hydrogen ion in water to produce acetic acid and the hydroxyl ion.
CH3COO–(aq)+HOH→CH3COOH+OH–(aq)
Water does not ionize acetic acid since it is a weak acid. The addition of the OH- ion, on the other hand, causes the solution’s basicity to rise. These salts are known as basic salts because of this. The pH of these salts’ aqueous solution is always above 7.
Salts of a Weak Base and a Strong Acid:
The acidic character of the salts generated by the neutralization reaction between weak bases and strong acids is discovered. Acidic salts include ammonium chloride and sodium chloride.
NH4Cl(aq)→Cl–(aq)+NH4+(aq)
The resulting ammonium ions react with the hydroxyl ions in the water molecule to generate ammonium hydroxide. As a result of the hydrogen ions produced, the solution becomes acidic. As a result, an aqueous solution of these salts is acidic, with a pH below 7.
NH+4(aq)+H2O⇋NH4OH(aq)+H+(aq)
Salts of Weak Base and Weak Acid:
Depending on the nature of the bases and acids involved, the salts formed by the neutralization reaction between a weak acid and a weak base might be slightly basic, mildly acidic, or neutral. The degree of water hydrolysis and ionization is unaffected by the solution’s concentration. CH3COONH4 is an example of such a salt. The following is the mechanism for the creation of such ions:
CH3COONH4++H2O⇋CH3COOH+NH4OH
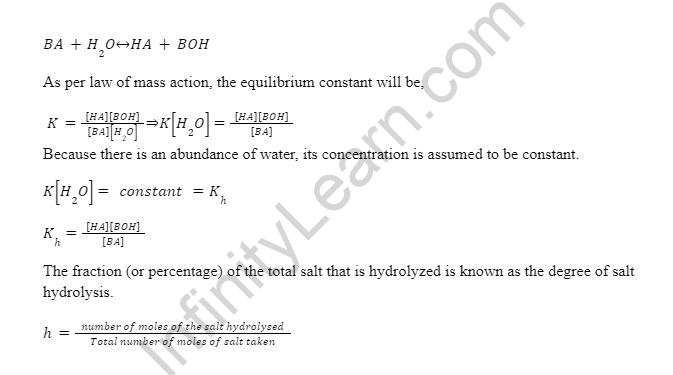
Salt Hydrolysis Formula
The hydrolysis constant (or hydrolytic constant) is the equilibrium constant obtained by applying the law of mass action to a hydrolysis reaction, and it is written as Kh.
Salt Hydrolysis pH Formula
The pH of such salts in an aqueous solution is expressed as
pH=7+12(pKa-pKb)
Thus, the pH is determined by the acids and base’s pKa and pKb, respectively.
Also read: Strength of Acid and Base
Frequently Asked Questions
What types of salts undergo hydrolysis?
Strong bases and weak acids produce salts that hydrolyze, resulting in a pH greater than 7. The anion in the salt is made up of a weak acid, most likely organic, that accepts the proton from the water in the process.
Why do salts undergo hydrolysis?
Whenever salt is introduced to water, the salt's cation, anion, or both ions react with the water, causing the solution to become acidic or basic. This is the hydrolysis process. A weak basic and acid solution is created when a cation of the salt combines with water.
Does sodium chloride undergo hydrolysis?
NaCl is a salt that contains a strong HCl acid and a strong base in the form of NaOH. There is no reaction between the NaCl salt ions and water since it does not undergo hydrolysis.







