Table of Contents
Introduction
The sector of interstitial compounds is mature, with a large body of literature. Nonetheless, a fundamental question such as the nature of their electronic structure is still the subject of lively (and sometimes heated) debate today. Furthermore, the industry has only scratched the surface of these materials’ myriad technical possibilities. Each year, people produce tones of the interstitial compound tungsten carbide for use in grinding and cutting tools, primarily due to its high hardness. Other properties, on the other hand, have seen little commercial application as of yet. To name a few, they are refractory while remaining metallic, and they frequently have abnormally low work functions, making them excellent electron emitters.
This combination suggests applications such as magnetohydrodynamic channel electrodes. Some interstitial compounds, like NBN, are superconducting, with transition temperatures second only to Nb3Sn. Tungsten carbide and related chemicals are moderately good catalysts that can operate in chemically hostile environments, such as those found in fuel cells. Many of them are good ferro and antiferromagnets. As we will try to demonstrate, this class of compounds offers both fascinating possibilities for technologists and real challenges for researchers.
Overview
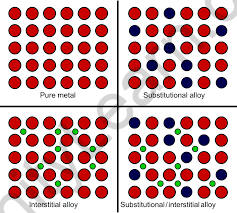
We realise that transition metals are the elements found in the periodic table’s d-block. Cobalt, nickel, iron, and other transition metals are examples. Transition metals can form complex non-stoichiometric compounds. These are compounds with arbitrary structure and proportions. Fe0.94O, for example, has a structure that is primarily due to the variable valency of transition elements. Non-stoichiometry is caused by defects in solid structures as well as the variable oxidation state of transition metals. These compounds do have a variable composition and are formed due to the variability of oxidation states and solid defects. Interstitial and nonstoichiometric compounds are sometimes the same.
Interstitial compounds
These are transition metal compounds formed when small atoms such as H, C, or N are trapped inside the interstitial vacant spaces in the metal’s crystal lattices. A few examples of interstitial compounds are TiC, TiH1.73, Mn4N, Fe3H etc.
- Because the transition metals’ vacant spaces are filled by small atoms, these compounds are hard and rigid.
- When during the formation of interstitial compounds, the chemical properties of the parent transition metals are not altered.
- However, physical properties such as density, rigidity, hardness, malleability, ductility, electrical conductivity, and so on change.
- Steel and cast iron are iron interstitial compounds formed with carbon. The malleability and ductility of iron are greatly reduced during the formation of these compounds, but the metal’s tenacity increases.
Interstitial compounds were first proposed in the late 1930s and are commonly referred to as Hagg phases after Hägg. Transition metals typically crystalise in either hexagonal close-packed or face-centered cubic structures, both of which are composed of layers of hexagonally close-packed atoms. There are two types of interstices, or holes, in both of these very similar lattices:
- Two tetrahedral holes per metal atom, implying that the hole is located between four metal atoms.
- One octahedral hole per metal atom, implying that the hole is located between six metal atoms.
An analysis of transition temperature measurements of pseudo-binary and ternary interstitial alloys reveals that there is no simple direct relationship between transition temperature and quantities such as valence electron-atom ratio, mass, and lattice volume. We have successfully systematised the experimental data into a general empirical model that can predict the most promising materials for achieving high transition temperatures.
Properties of interstitial compounds
- The interstitial compounds have the same chemical properties as the parent transition metals.
- They are hard and exhibit metallic properties such as electrical and thermal conductivity, lustre, and so on.
- Since metal-nonmetal bonds are stronger in interstitial compounds than metal-metal bonds in pure metals, the compounds have much higher melting points than pure metals.
- They are said to be less dense than the parent metal.
- Interstitial compounds having hydrogen (i.e., metal hydrides) are potent reducing agents.
- Carbon-containing compounds, which behave as carbides, are chemically inert and extremely hard, similar to diamond.
- Malleability and ductility are altered in these compounds. Steel and cast iron are two examples.
Transition elements form interstitial compounds
The transition elements combine to form a wide range of interstitial compounds. Small atoms such as hydrogen, carbon, boron, and nitrogen fill the empty spaces in these compounds’ lattices. The small atoms enter the voids or interstitial sites between the crystalline metal’s packed atoms. We can say that, they are typically non-stoichiometric and neither ionic nor covalent. The expressions of non-stoichiometric compounds do not correspond to any of the metal’s normal oxidation states. These compounds are known as interstitial compounds because of the unique properties of their composition. These compounds are harder than pure metals and have higher melting points. They are chemically inert and retain metallic conductivity. The presence of small atoms reduces the malleability and ductility of metals while increasing their tensile strength.
Interstitial compounds also can have semi conductivity, fluorescence, and act as heterogeneous catalysts. Their catalytic activity is associated with the variable oxidation states of d-block elements and their compounds, as well as their ability to form interstitial compounds that can absorb and activate the reacting species.
Interstitial compounds can be formed by elements from the 3d-transition series, such as Ti2C, V2C, ScN, TiN, Fe4N and so on. These compounds have alloy properties such as hardness and conductivity. These elements can also combine to form non-stoichiometric compounds.
FAQ’s
Q. What do you mean by interstitial compounds?
Ans: Generally, an interstitial compound also known as an interstitial alloy, is a compound formed when an atom with a small enough radius sits in a metal lattice’s interstitial “hole.” Small atoms include hydrogen, boron, carbon, and nitrogen.
Q. What are the properties of interstitial compounds?
Ans: They are tough and have excellent heat and electricity conductors. They have the same chemical properties as the parent metal. They have higher melting points than pure metals.
Q. What is meant by non-stoichiometric compounds?
Ans: Non – stoichiometric compounds are said to be chemical compounds that deviate from stoichiometry in the sense that their elemental composition cannot be represented by a ratio of well-defined natural numbers and thus violate the law of definite proportions.






