Table of Contents
Silicon is a representative of the carbon family and a non-metallic chemical element with the atomic number 14 that belongs to group 14, period 3 in the periodic table’s p-block. Carbon is above it, while germanium, tin, lead, and flerovium are beneath it. Silicon is a metallic element, one of seven elements that have both nonmetal and metallic properties depending on the other element with which it combines. Silicon, which is used in electronics, acts like a metal, whereas glass, a silicon compound, has non-metallic properties. This element is denoted by the symbol ‘Si.’ Silicon’s electronic configuration is (Ne) 3s² 3p². At 20°C, silicon is in a solid-state. It is used in the production of moulding compounds.
Overview
Silicones are polymers also referred to as polysiloxanes. These are the polymers that include any inert, synthetic compound composed of iterative siloxane units. It consists of an alternating chain of oxygen and silicon atoms that are frequently combined with hydrogen and carbon. Silicones are the current synthetic object class, and they contribute to thousands of applications that provide safety and well-being in everyday life. Silicones are a broad class of high-performance materials that include silicone fluids, silicone polymers, and reactive silanes. These materials are widely used in a wide range of industrial and consumer products, and they provide critical benefits in a variety of fields such as personal care, health care, aerospace, transportation, electronics, and construction.
Silicones have distinct properties among polymers due to the presence of organic groups attached to a chain of inorganic atoms. They have been used in a variety of industries, including electronics, paints, construction, and food.
The information about silicones from various chemistry-related articles is available here. Silicones are polymers also referred to as polysiloxanes. These are the polymers that include any inert, synthetic compound composed of iterative siloxane units. Students who want to flourish in chemistry need to be well known about silicones to get deep knowledge about it to do well on their exams. The definition, colour, types and uses are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional chemistry help.
Silicones
Silicones are polymers with a silicon-oxygen backbone similar to silicon dioxide (silica) but with organic groups attached to the silicon atoms via C-Si bonds. Organic groups are exposed to the outside due to the silicone chain.
Finally, despite having a very polar chain, silicones have physical properties similar to those of an alkane. Because the Si-O bond energy is much greater than the C-C bond energy, the silicone’s -Si-O-framework provides thermal stability, as in silica, allowing the polymers to be used where comparable organic materials would melt or decompose.
To differentiate between silicones, systematic names based on the monomer are used. Silane, SiH4, is the most basic silicon compound and belongs to the homologous series of silanes. Silanes are alkanes, the most basic of which is methane, CH4.
The existence of oxygen atoms in the silicone chain is indicated by the systematic name siloxane, which is so named because it contains a silicon atom, an oxygen atom, and is saturated like an alkane.
If the groups attached to the siloxane chain are phenyl groups, the resulting silicone is known as poly (diphenyl siloxane) and contains these repeating units along the chain.
Silicones with methyl groups along the backbone are the most commonly used. By substituting other organic groups for the methyl groups, properties such as solubility in organic solvents, water repellence, and flexibility can be altered. Silicones with phenyl groups, for example, are more flexible polymers than those with methyl groups. They are also superior lubricants and solvents for organic compounds.
The structure of silicone repeating units can be represented as follows:
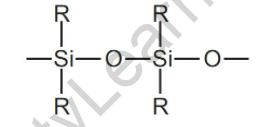
R denotes organic groups attached to the silicone backbone.
Colour of Silicon
Plain silicon is a tough, dark grey solid with a metallic luster and an octahedral crystalline structure similar to that of diamond, with which silicon shares many chemical and physical properties.
Types of Silicone
(1) Liner silicones:
They are made by hydrolyzing and then condensing dialkyl or diaryl silicon chlorides.
- Silicone rubbers: These silicones are held together by methylene or other similar groups.
- Silicone resins: These are created by combining silicones with organic resins such as acrylic esters.
(2) Cyclic silicones:
A cyclic silicone is a compound with a cyclical structure rather than the chain structures found in dimethyl silicones.
(3) Cross-linked silicones:
Once monoalkylchloro silanes are hydrolyzed, followed by a polymerization reaction, a very complex structure known as the crosslinked polymer is formed.
Use of Silicon
- Silicones can exist in a variety of states, from liquids to solids, allowing engineers, inventors, and businesses to use them as a critical component in a wide range of industrial applications. Because of their versatility, silicones are an important component of products that improve our lives, whether as rubbers, fluids, resins, silicone gels, or silicon glue. Silicones can be found in a wide range of applications, from computers and engineered spacecraft to shampoo and baking moulds. Silicones can also be used in renewable energy, such as wind turbines and solar panels, which rely on silicone technology.
- Silicones are broadly used in cosmetics and personal care products. They are used in deodorants to reduce the white residue and tacky feel of antiperspirants. Silicones are used in cosmetics, shampoos, and conditioners to keep the compound’s colour and luster. Silicones are said to provide better shine, and skincare products can be made with higher SPF.
- Silicones are widely used in the construction of commercial and residential structures. They are said to protect against moisture and bacterial growth.
- Keypads, keyboards, and copier rollers are all made of silicone. Silicones are used in many other components of computers, mobile electronics, and home entertainment equipment. Silicones make LED lighting technology possible. It has excellent dielectric properties as well as high thermal stability. As a result, it is used in a wide range of electrical transmission applications.
- Silicones are excellent materials for increasing the efficiency, durability, and performance of solar panels and photovoltaic devices. They can withstand the sun for years on end.
- Silicone adhesives and sealants are widely used to seal and protect many parts of an aeroplane due to their high stress and temperature resistance. It’s being used to seal windows, doors, overhead bins, fuel tanks, engine gaskets, hydraulic switches, wings, wing edges, landing gear, electrical devices, vent ducts, and even black boxes.
- Silicone bakeware and cookware are widely available and used in the kitchen to prepare a variety of dishes. These utensils have no effect on the taste or quality of food.
- There are silicone-enhanced paints on the market today. These paints aid in the flexibility of the exterior coatings of houses, bridges, and railway cars, allowing them to withstand different temperature cycles or weather conditions and, in particular, preventing cracking. Silicone coatings are less prone to corrosion.
- Goggles and diving masks are made of silicone. Silicones are a lightweight, long-lasting, water-resistant, and high-performance material. Silicones with such properties can be used to create new sportswear and goods.
- Silicones have been used in the manufacture of a variety of toys.
- Silicones are widely used as lubricants. Airsoft gun parts, bicycle chains, and other mechanisms are lubricated with silicone greases.
Also read: Important Compounds of Silicon
FAQs
Q. Is silicone toxic to humans?
Ans: In general, siloxanes (silicones) are well tolerated by the human body, and thus they are an essential component of novel treatment, health care, and nursing methods. These are commonly thought to be non-toxic to humans and the environment or only slightly toxic.
Q. What is silicone made of?
Ans: Although the main chain of most organic synthetic polymers is made up of repeating carbon (C) atoms, silicone is an “inorganic synthetic polymer” whose main chain is made up of polysiloxane, which is the repetition of silicon (Si) and oxygen (O) atoms.
Q. Is silicone plastic or rubber?
Ans: Basically, silicone is a member of the rubber family. However, if you define plastics broadly, as we do, silicone is a cross between synthetic rubber and a synthetic plastic polymer. Silicone can be used to create malleable rubber-like substances, hard resins, and spreadable fluids.







