Table of Contents
Introduction
Calorimetry is a method for estimating the quantity of heat involved in a chemical or physical reaction, as well as the heat transported to or from a substance. A calibrated and insulated device known as a calorimeter is used to exchange heat. Calorimetry experiments are conducted under the assumption that no heat exchange occurs between the insulated calorimeter and the surrounding environment. A well-insulated calorimeter inhibits heat transfer between the calorimeter and its external environment, essentially limiting the calorimeter’s “surroundings” to no system components (and the calorimeter itself). This allows for precise measurement of heat in chemical processes, such as determining the energy content of foods.
The information about calorimetry from various physics-related articles is available here. Calorimetry and its types are important topics in physics. Students who want to flourish in physics need to be well known about calorimetry to get deep knowledge about it to do well on their exams. The definition, diagrams, working, and types are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional physics help.
Overview
The quantity of heat transmitted by the process under investigation is calculated using the temperature change observed by the calorimeter. A system is defined in a calorimeter as the substance or substances having undergone the chemical or physical modification, or in other words, the reaction, and the surroundings are all other matter, including the solution and any other components in the calorimeter that either provide or soak up heat from the system.
Consider a basic example that shows the core notion of calorimetry before moving on to the calorimetry of chemical reactions. Assume that a hot piece of metal is immersed in a low-temperature substance, such as cool water. The hot metal will transfer heat to the water. The temperature of the metal will drop, while the temperature of the water will rise, until the two substances reach thermal equilibrium, at which point they will be at the same temperature. When this happens in a calorimeter, 100% of the heat is passed between the two substances, with no heat gained or lost by the surroundings.
Calorimeter: Diagram and Working
A calorimeter is a gadget or device used for heat measurements necessary for calorimetry. It primarily consists of a metallic vessel made of materials such as copper and aluminium that are good conductors of electricity. There is also a feature for stirring the contents of the vessel. This steel jar with a stirrer is encased in an insulating jacket to reduce heat loss to the environment. To monitor the change in thermal properties within, a thermometer can only be inserted through one aperture.
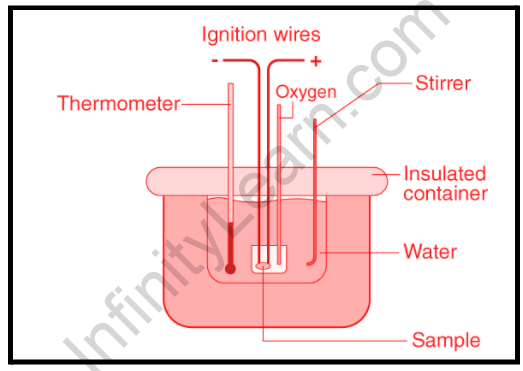
This makes it simple to take such measurements. Assume a set amount of fuel is burnt in a calorimeter. Water is sprayed into the container, and the fuel is burned, leading to water heating. The heat obtained by the water is equal to the heat lost by the fuel. We can say that this is the reason why it is critical to isolate the calorimeter from the environment in order to improve the experiment’s accuracy. A thermometer can then be used to determine how much heat has changed. This approach may be used to calculate the heat capacity of water as well as the energy stored inside a fuel.
Calorimeters have two vessels: an exterior and an inner vessel. Because the air between them acts as a thermal insulator, there is little to no heat transfer between the inner vessel’s contents and the outer environment. To keep the inner vessel in the middle of the outer vessel, lab calorimeters use a fiber ring constructed of insulating material. A thermometer in the inner container measures the temperature of the liquid, and a stirrer stirs the liquid to distribute the heat throughout the container.
Whether there is an exothermic reaction, one that emits thermal energy through heat or light, inside the solution in the calorimeter, the temperature goes up. Heat is lost and the temperature drops in an endothermic reaction, which drains thermal energy from the environment. The substances are in thermal equilibrium if they do not transmit energy between them. The mass and specific heat of the solution, as well as the temperature differential, can be used to calculate how much heat the reaction uses.
When compounds A and B react, the temperature change is used to determine the enthalpy change per mole of substance A. The formula is:
q = Cv(Tf – Ti )
Q = quantity of heat in joules.
Cv =calorimeter’s heat capacity in joules per Kelvin (j/K)
Tf = final temperature and Ti is said to be the initial temperature.
Constant-Volume Calorimetry
Constant Volume Calorimetry, often known as bomb calorimetry, is a technique for determining the heat of a process while maintaining a constant volume and resisting high pressure. Although these two features of bomb calorimetry contribute to the difficulties of bomb calorimetry, they also contribute to the accuracy of the results.
Calorimetry is being used to quantify quantities of heat and can be used to determine the heat of a reaction through experiments. A coffee-cup calorimeter is commonly employed since it is less complicated than a bomb calorimeter, yet constant volume or bomb calorimetry is suitable for measuring the heat evolved in a combustion reaction. A constant volume calorimeter is also more precise than a coffee-cup calorimeter, but it is more difficult to use since it necessitates a well-built reaction container that can tolerate high-pressure changes, which are common in chemical reactions.
The majority of serious calorimetry done in research labs includes determining temperatures of combustion, which are necessary for determining standard enthalpies of the creation of the thousands of novel compounds created and characterized every month. The system is a constant volume calorimeter that is sealed or isolated from its surroundings, which explains why its volume is stable and no volume-pressure work is performed.
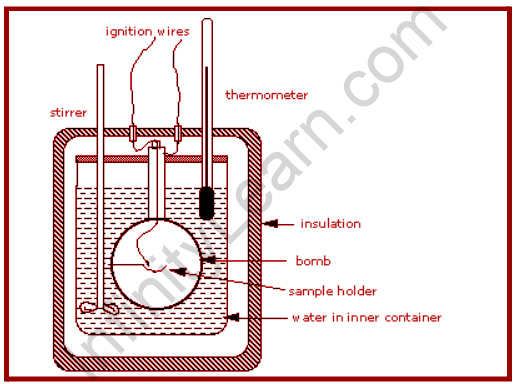
The reaction container must be built to withstand the high pressure generated by the combustion process, which corresponds to a contained explosion because the process takes place at constant volume. The vessel is sometimes referred to as a “bomb,” and the technique is referred to as bomb calorimetry. The reaction starts when a capacitor is discharged across a tiny wire, igniting the mixture.
Constant-Pressure Calorimetry
The change in enthalpy of a reaction happening in a liquid solution is measured by a constant-pressure calorimeter. In this instance, the gaseous pressure above the solution remains constant, and the reaction is said to be occurring under constant pressure circumstances. The change in enthalpy is equal to the heat transferred to/from the solution in order for the reaction to occur, and a constant-pressure calorimeter detects this heat of reaction. Because a bomb calorimeter has a constant volume, there is no pressure-volume work involved, and the heat measured is proportional to the change in internal energy.
A coffee-cup calorimeter is a small example of a constant-pressure calorimeter, which is made up of two nested Styrofoam cups and a cover with two holes for inserting a thermometer and a stirring rod. The inside cup contains a predetermined amount of liquid, usually water, that absorbs the heat generated by the reaction. The outer cup is assumed to be completely adiabatic, which means it does not absorb any heat. As a consequence, it’s considered that the outer cup is a perfect insulator.
Also read: Important Topics Of Physics: Isobars and Isotopes
Frequently Asked Questions (FAQs)
What do you mean by calorimetry?
Calorimetry is a method for estimating the quantity of heat involved in a chemical or physical reaction, as well as the heat transported to or from a substance. A calibrated and insulated device known as a calorimeter is used to exchange heat.
What is the device used for measuring calorimetry?
Generally, a calorimeter is a tool or device that is being used to measure calorimetry. The calorimeter is made up of a metallic vessel and a stirrer. Both the vessel and the stirrer are constructed of the same material, which is copper or aluminium. The vessel is housed in a wooden jacket with a small aperture in the outer jacket through which a mercury thermometer can be placed.
What is an example of constant pressure calorimetry?
A coffee-cup calorimeter is a small example of a constant-pressure calorimeter, which is made up of two nested Styrofoam cups and a cover with two holes for inserting a thermometer and a stirring rod.






