Table of Contents
Introduction:
Physics is the science of measuring temperatures, movements, and other physical properties of systems and objects. It can be applied to single-celled organisms, mechanical systems, galaxies, stars, and planets, as well as the processes that regulate them. Thermodynamics is a discipline of physics that studies the interactions between heat energy and other types of energy. It explains how thermal energy is turned into different types of energy and how it impacts matter.
When scientists investigate isothermal processes in systems, they look at the relationship between heat and energy, as well as the mechanical energy required to change or maintain a system’s temperature. This knowledge aids biologists in their research into the regulation of temperature in living organisms. It’s also used in planetary science, space science, engineering, geology, and a variety of other fields.
Isothermal processes
A thermodynamic process in which the temperature of a system remains constant is known as an isothermal process. Thermal equilibrium is maintained because heat is transferred into and out of the system at such a sluggish rate. The change of a substance, object, or system at a constant temperature is known as the isothermal process. Typically, there are two types of occurrences that can cause this process to occur. If a system comes into contact with a thermal reservoir from the outside, the system gradually adjusts its temperature to that of the reservoir through heat exchange to preserve thermal equilibrium. In another phenomenon, there is no heat transfer between a system and its surroundings. The temperature of the system is altered during this procedure to keep the heat consistent. The Adiabatic Process is the name given to this process.
Isothermal and Adiabatic Processes: What’s the Difference?
An isothermal process occurs at a constant temperature, but other system parameters can be modified to suit the situation. In an adiabatic process, on the other hand, heat transfer occurs to maintain a constant temperature. The major distinction between isothermal and adiabatic processes is that the former happens at a constant temperature, whilst the latter occurs at a variable temperature. The work done in an isothermal process is caused by a change in the system’s net heat content. The work done in an adiabatic process, on the other hand, is due to a change in its internal energy.
What is Boyle’s Law?
For perfect gases, an isothermal process is very interesting. An ideal gas is a hypothetical gas in which the molecules do not interact and collide elastically. The internal energy of a fixed amount of an ideal gas is only affected by temperature, according to Joule’s second law. In an isothermal process, the internal energy of an ideal gas is thus constant.
The product of Pressure and Volume (PV) is constant in an isothermal environment for an ideal gas. Boyle’s law is the name for this. Robert Boyle, a physicist and chemist, published this law in 1662. Because French physicist Edme Mariotte independently discovered the same concept in 1679, Boyle’s law is also referred to as Boyle–Mariotte law, or Mariotte’s law.
Boyle’s Law Equation:
If the temperature and amount of gas in a closed system stay constant, the absolute pressure exerted by an object of an ideal gas is inversely proportional to the volume it occupies.
The above-mentioned law can be expressed in a few different ways. The simplest method is as follows:
PV = k
P denotes pressure, V denotes volume, and k denotes a constant.
The law can also be used to find the volume and pressure of a system when the temperature is held constant in a system as follows:
PiVi = Pf Vf
where,
Pi is the initial pressure
Pf is the final pressure
Vi is the initial volume
Vf is the final volume
Boyle’s Law explains how humans breathe and expel air out of their lungs. Lung volume reduces and increases as the diaphragm tightens and expands, changing the air pressure inside them. Inhalation or exhalation is caused by a pressure differential between the interior of the lungs and the outside air.
Examples of Isothermal Process:
- In systems with some mechanisms of temperature regulation, an isothermal process occurs. This occurs in a wide range of systems, from highly organised machinery to living cells. Below are a few instances of isothermal processes.
- Isothermal processes include changes in the state or phase of various liquids during the melting and evaporation processes.
- The Carnot engine is an example of an industrial application of the isothermal process. Some of the cycles in this engine are carried out isothermally.
- A refrigerator works on the principle of the isotherm. A refrigerator’s mechanism undergoes a series of adjustments, but the temperature inside remains constant. The heat energy is extracted and transferred to the surrounding environment at this point.
Define Isothermal Process
The “isothermal process,” is a thermodynamic process in which a system’s temperature does not change. Thermal equilibrium is maintained because heat is transferred into and out of the system at such a sluggish rate. The term “thermal” refers to the heat produced by a system. Because “iso” means “equal,” “isothermal” means “equal heat,” which is how thermal equilibrium is defined.
Isothermal Processes and States of Matter:
Isothermal processes can take numerous forms. Evaporation of water into the air, as well as boiling of water at a specified boiling point, are examples. Many chemical reactions also preserve thermal equilibrium, and in biology, a cell’s interactions with its surrounding cells (or other matter) are referred to as an isothermal process.
“Phase alterations” include evaporation, melting, and boiling. That is, they are temperature and pressure-controlled changes in water (or other fluids or gases)
What exactly is an Isotherm?
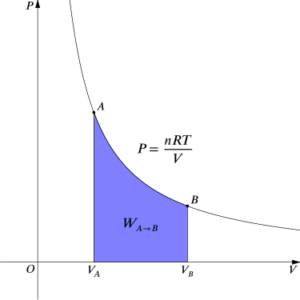
All the curves that represent two states of a system where the temperature is the same during an isothermal process are called isotherms if we observe the relationship between temperature and any other thermodynamic variable such as pressure, volume, and others and draw a graph on the Cartesian plane.
An isotherm is a set of curves or lines that represent two states of a system at the same temperature in an isothermal process.
For example, during an isothermal process, a line drawn as illustrated in the image below has two states A and B, both of which are at the same temperature, hence this line is called isotherm. On the X-axis, we can examine any additional thermodynamic variable.
Isothermal Process Diagram:
In physics, diagrams are used to depict such reactions and processes (graphs). An isothermal process is depicted in a phase diagram by following a vertical line (or plane in a 3D phase diagram) along with a constant temperature. To keep the system’s temperature constant, the pressure and volume can alter.
Even if the temperature does not vary, it is possible for a substance to change its state of matter. As a result of the evaporation of water as it boils, the temperature remains constant as the system’s pressure and volume fluctuate. This is then graphed, with the temperature remaining constant throughout.
What All of This Means:
When scientists look at isothermal processes in systems, they’re really looking at heat and energy, and how they relate to the mechanical energy required to change or maintain a system’s temperature. This knowledge aids biologists in their research into how living things regulate their temperatures. It’s also used in engineering, space science, planetary science, geology, and a variety of other fields. Heat engines are based on the concept of thermodynamic power cycles (and thus isothermal processes). Humans use these devices to power electrical producing plants as well as cars, trucks, planes, and other vehicles, as noted above. Such systems can also be found on rockets and spacecraft. Engineers use thermal management methods (also known as temperature control) to improve the efficiency of machines.
Frequently Asked Question (FAQs)
Question: Is it true that the Isothermal process involves heat transfers?
Answer: Yes, but the question now is why and how.
Let’s look at an example of a piston-cylinder to see how it works.
If heat is applied to the cylinder’s bottom. The piston will move while the temperature is kept constant. Either a compression or an expansion procedure is used. The heat is transmitted, but the temperature of the system remains unchanged. This is why heat is injected at a consistent temperature during the Carnot cycle.
Question: Why is the isothermal process so slow?
Answer: The Isothermal process must proceed at a slow pace. As you can see, keeping the system’s temperature consistent allows for heat transfer. It denotes that the system and the body are in thermal balance.
Question: What is the definition of an isothermal process?
Answer: An isothermal process is a thermodynamics process in which the entire temperature of a system remains constant until the process is done.
Question: At constant (A) temperature, (B) volume, and (C) heat, an adiabatic process occurs (D) None of the aforementioned.
Answer: Since isothermal operations take place at a constant temperature, the answer is yes. Isochoric refers to a process that occurs at a constant volume magnitude. All thermodynamic processes that occur at constant heat are referred to as adiabatic processes, hence (C) Heat is the right answer.







