Table of Contents
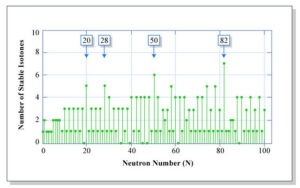
Isotones: Any of two or more types of atoms or nuclei with much the same number of neutrons is called an isotone. Because the nucleus of this chlorine species has 17 protons and 20 neutrons, the nucleus of this species of potassium has 19 protons and 20 neutrons, and chlorine-37 and potassium-39 are isotones. Isotones 50 (five: 86Kr, 88Sr, 89Y, 90Zr, 92Mo) and 82 have the greatest number of observationally stable nuclides (six: 138Ba, 139La, 140Ce, 141Pr, 142Nd, 144Sm). There seem to be no permanent isotones for neutron values 19, 21, 35, 39, 45, 61, 89, 115, 123, and 127 or more. On the other hand, the proton numbers for which there are no steady isotopes are 43, 61, and 83 or more.
A Brief Outline
Isotopes, isobars, and isotones are terminology used to describe how atoms of different chemical elements interact. Isotopes, isobars, and isotones are atoms with the same number of protons but different numbers of neutrons. Isobars are atoms of different chemical elements with equal atomic mass values, whereas isotones are atoms of different chemical elements with an equal number of neutrons in the atomic nucleus. Isobars are elements with the same number of nucleons as each other (sum of protons and neutrons). All the above-mentioned elements have the same number of protons and neutrons in their nuclei but different figures of protons and neutrons.
Define Isotone:
Atoms in diverse combinations make up all matter. Protons, neutrons, and electrons constitute an atom. Each proton carries a charge of; each electron carries a charge of, so each neutron carries no charge. It’s useful for nuclear physicists to look at nuclei with the same number of neutrons while studying different nuclear species, yet changing the number of protons impacts the chemical makeup of the nucleus. Isotones aren’t as well-known as the closely related notion of isotope, which has the same number of protons and a variable number of neutrons. Isotone has a neutron, whilst isotope has a p for proton, as a mnemonic for remembering the two ideas.
Isotone example:
40Ca20 and 39K19 have 20 neutrons, indicating that calcium and potassium have the same number of neutrons. As a result, they are isotones. Another example is that silicon and phosphorus both have the same number of neutrons (16), but their mass numbers (30, 31) and atomic numbers (14, 15) are different. The numbers of found natural isotones provide information about a neutron configuration’s stability.
Important concepts
Neutrons, isotopes, isobars, and isotones were discovered.
The presence of neutrons was not discovered until 1932, which is a stunning discovery. The most common atomic imagination at the moment is protons and electrons. Rutherford’s alpha scattering studies revealed that an element’s atomic mass number, which is ‘A,’ is slightly larger than twice its atomic number, which is ‘Z,’ for the majority of atoms, and that all of an atom’s mass is concentrated in a tiny space in the center of the atom. Proof of this can be found in the alpha particles, which turned 180 degrees.
Until 1930, certain electrons in the dense nucleus were assumed to coexist with protons, despite the enormous amount of energy required to sustain such a system, which was much beyond atomic energies. If we use a size of 0.2 nanometers for the hydrogen atom, the electron confinement energy is 38eV, which is the precise magnitude of the atomic electrons. If the electron coexisted with protons in the nucleus, the electron confinement energy is estimated to be around 250 Mev! The 38eV is a few orders of magnitude higher.
Isotones 50 (which have five stable nuclides: 88Sr, 86Kr, 90Zr, 89Y, and 92Mo) and 82 have the biggest numbers of stable nuclides (six: 139La, 138Ba, 141Pr, 140Ce, 144Sm, and 142Nd). Where there are no stable isotones, the neutron counts are 19, 21, 35, 39, 45, 61, 89, 115, and even higher. On the other hand, Proton numbers are reported as 43, 61, and 83 or even higher if there are no stable isotopes.
This has something to do with nuclear magic numbers, the number of nucleons in the nucleus that form complete shells. For instance, 2, 8, 28, 50, and 82. Except for 1 (which means 2H and 3He), 5 (which means 9Be and 10B), 55 (the 97Mo and 99Ru), and 107, no more than one stable nuclide has an odd neutron number (the 179Hf and 180mTa). The odd neutron numbers of a primitive radionuclide and a stable nuclide are 27 (50V), 65 (113Cd), 81 (138La), 85 (147Sm), and 105. (176Lu). Where there are two primordial radionuclides, the neutron numbers are 88 (151Eu and 152Gd) and 112 (151Eu and 152Gd) (187Re and 190Pt).
Significance of isotones in the NEET exam
To ace the NEET exam, you must grasp all of your topics’ fundamental concepts. For this, answers must be greatly simplified and presented in a comprehensible manner, using relatively simple procedures and fewer calculations. This allows you to save time and effort during the exam. For online learning and understanding of concepts, live classes are available. Simple free pdf is also available in offline mode. As a result, learning and taking notes happen at the same time. You can get a complete set of notes by accessing these free PDFs.
Also read Important Topics Of Physics: Isobars and Isotopes.
FAQs (Frequently asked questions)
What are some examples of isotones?
Carbon and oxygen were two separate atoms with comparable numbers of neutrons (eight), but differing numbers of protons (six and eight, respectively).
Who was the first to discover the Neutron?
A breakthrough occurred when it was demonstrated that bombarding Beryllium with alpha particles from that radioactive source produced penetrating but non-ionizing radiation. Scientists were perplexed by this sort of neutral radiation because photons were the only acknowledged neutral radiation. And, if it had been a photon, the neutral radiation would have exited/cleared the beryllium atom with significantly more energy than it normally does.
How do you put Binding Energy and Binding Energy per Nucleon into statements?
The energy required to shatter a nucleus into its constituent particles is referred to as binding energy (neutrons and protons). The nucleus is fractured in such a way that the constituent particles are separated by a large distance, preventing them from interacting with one another. Binding energy per nucleon is the average energy required to remove one nucleon from the nucleus.





