Table of Contents
Introduction: Size of Nucleus
Ernest Rutherford’s efforts to test Thomson’s “plum pudding model” of the atom resulted in the discovery of the nucleus in 1911. J.J. Thomson had already discovered the electron. J.J.Thomson reasoned that because atoms are electrically neutral, there must be a positive charge as well.
Thomson proposed that an atom was made up of negative electrons randomly scattered within a positive charge sphere in his plum pudding model. Ernest Rutherford later devised an experiment, with the assistance of Ernest Marsden and his research partner Hans Geiger, that involved the deflection of alpha particles (helium nuclei) directed at a thin sheet of metal foil. He reasoned that if J.J Thomson’s model was correct, positively charged alpha particles would easily pass through the foil with little deviation in their paths, because the foil should act as electrically neutral if the negative and positive charges are so intimately mixed as to appear neutral. Many of the particles, much to his surprise, were deflected at very large angles. Because an alpha particle’s mass is approximately 8000 times that of an electron, it became clear that a very strong force was required to deflect the massive and fast-moving alpha particles. He realized that the plum pudding model could not be correct and that the alpha particle deflections could only be explained if the positive and negative charges were separated and the mass of the atom was a concentrated point of positive charge. This provided support for the concept of a nuclear atom with a dense center of positive charge and mass.
Overview
Rutherford’s experiment allowed us to determine the size of the nucleus. By determining the point of closest approach of an alpha particle, we can calculate the size of the nucleus. The point of closest approach was estimated to be 4×10-14mby shooting alpha particles with the kinetic energy of 5.5 Me V. There is no contact because the repulsive force acting here is Coulomb repulsion. This means that the nucleus is smaller than 4×10-14 m in size.
After many more iterations of the experiment, the sizes of the nuclei of various elements have been precisely measured. Following this, a formula for calculating the size of the nucleus was developed.
R=R0 A1/3
Where,
R0=1.2×10-15m
We can deduce from the formula that the volume of the nucleus, which is proportional to R3, is proportional to A. (mass number). Another thing to notice about the equation is that there is no mention of density. This is because the density of the nuclei does not vary with the element.
The nucleus has a density of about 2·3×1017 kg·m-3.
The size of the nucleus is a fundamental physical property of an atom. It is defined as the smallest unit in which an atom can be found.
Composition and size of the nucleus
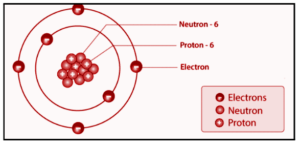
Atom nuclei contain protons and neutrons, while electron clouds surround the nuclei and contain electrons. In an atomic nucleus, positively charged protons are very close together, and the repulsive force of like charges is enormous. The strong force is a type of interaction that holds protons and neutrons together. The positively charged protons would blow the nucleus apart if not for the strong force. A densely packed combination of protons and neutrons makes up an atom’s nucleus. Because these are the two heavy particles in an atom, 99.9% of the mass is concentrated in the nucleus. The nucleus of an atom is positively charged generally because protons have a net positive charge, whereas negatively charged electrons rotate around the core nucleus.
The nuclear forces that hold protons and neutrons together are strong because the mass concentration at an atom’s nucleus is massive. The nucleus’s composition can be described by two main hypotheses: the proton-neutron hypothesis and the proton-electron hypothesis. The atom’s nucleus contains protons and neutrons, while electrons revolve around the nucleus. Positively charged protons are close to each other in the atomic nucleus. At the same time, the repulsive forces are massive. Strong forces of attraction cause protons and neutrons to bind. Positively charged protons will be blown away in the absence of stronger forces.
Nucleus size
The nucleus has a diameter of 1.6 fm (10-15 m) for the lightest atoms and 15 fm for the heavier atoms. The nucleus of an atom is substantially smaller than the nucleus of an atom. Despite its smaller size, it has the most mass of any atom. The nucleus of an atom is the focal point of the atom, where the majority of its mass is concentrated. We discovered that the nucleus of an atom contains a larger portion of the atom’s mass thanks to Rutherford’s study of the dispersion of alpha particles. In terms of numbers, the nucleus of an atom has nearly 10-14 times the volume of the atom while retaining 99.99 percent of the atomic mass.
Except for a small amount of mitochondrial DNA and, in plant cells, plastid DNA, the cell nucleus contains the entire genome. To form chromosomes, nuclear DNA is organized as multiple long linear molecules in a complex with a wide range of proteins such as histones. The genes on these chromosomes are organized in such a way that they promote cell function. The nucleus maintains the integrity of genes and controls the activities of the cell by regulating gene expression; as a result, the nucleus serves as the cell’s command and control center.
Diameter of nucleus
The diameter of the nucleus ranges from 1.70 fm 1.70×10-15mfor hydrogen (the diameter of a single proton) to approximately 11.7 fm for uranium. These dimensions are approximately 26,634 times smaller than the diameter of the atom itself (nucleus + electron cloud) (uranium atomic radius is approximately 156 pm 156×10-12m to approximately 60,250 (hydrogen atomic radius is about 52.92 pm). The neutron has a positively charged core with a radius of 0.3 fm that is surrounded by a compensating negative charge with a radius of 0.3 fm to 2 fm.
The proton has a positive charge distribution that decays approximately exponentially, with a mean square radius of about 0.8 fm. Spherical, rugby ball-shaped (prolate deformation), discus-shaped (oblate deformation), triaxial (a combination of oblate and prolate deformation), or pear-shaped nuclei are all possible.
Atomic nucleus radius
An atom is made up of a nucleus in the center surrounded by electrons that revolve around the letter t. The nucleus is made up of protons (positively charged particles) and neutrons (neutrally charged). The nucleus is extremely small in comparison to the size of the atom. The radius of an atom is =10-10m An atomic nucleus has a radius of =10-15m
A chemical element’s atomic radius is a measurement of the size of its atoms, usually the mean or typical distance from the center of the nucleus to the boundary of the surrounding electron shells. Because the boundary is not a well-defined physical entity, various non-equivalent definitions of atomic radius exist. Van der Waals radius, ionic radius, metallic radius, and covalent radius are four commonly used definitions of atomic radius.
The radii of isolated neutral atoms are typically defined as being between 30 and 300 pm (trillionths of a metre), or between 0.3 and 3 ngströms. As a result, the radius of an atom is greater than 10,000 times the radius of its nucleus (1–10 fm) and less than 1/1000 of the visible light wavelength (400–700 nm).
A nucleus’ radius is in the order of 10-13cm. Ernest Rutherford discovered the atomic nucleus, a small, dense region of protons and neutrons at the center of an atom, in 1911 based on the 1909 Geiger–Marsden gold foil experiment.
The basic building block of chemistry is an atom, which is the smallest unit of any matter and has the properties of a chemical element. The majority of an atom’s interior is empty space, with its centre containing positively charged protons and neutral neutrons. Protons and neutrons make up the nucleus of an atom. The nucleus is surrounded by electrons, which are negatively charged particles that form a cloud around it. In this article, we’ll look at the theory behind nucleus size and see what the Rutherford gold foil experiment revealed.
Also read: Important Topic Of Physics: Hydrogen Spectrum
FAQs
What exactly is the nucleus?
The nucleus is a double-membraned organelle that contains genetic material as well as other instructions needed for cellular processes. It is found only in eukaryotic cells and is one of the largest organelles.
What is Found in an Atom's Nucleus?
The nucleus, the atom's thick central core, contains both protons and neutrons. In terms of energy levels, electrons are found outside the nucleus. Neutrons are neutral, while protons have a positive charge and electrons have a negative charge.





