Table of Contents
Introduction: Isobars and Isotopes
Subatomic particles protons, electrons, and neutrons make up atoms, which are the smallest particles of matter. Protons have a positive electric charge and a stable mass, while neutrons have a mass similar to the proton but do not carry an electric charge. Electrons are negatively charged and are the main carriers of electricity in solid substances. Generally, matter is any substance that has mass and takes up space.”
Overview
The information about isobar isotopes from various physics-related articles are available here. Isobars and Isotopes are important topics in physics. Students who want to flourish in physics need to be well known about isotopes and isobars to get deep knowledge about it to do well on their exams. The definitions, brief explanations, and differences are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional physics help.
We can say that isobars are elements with dissimilar chemical properties but identical physical properties. So, we can define isobars as elements with various atomic numbers but the same mass number. Due to the difference in the number of electrons, their chemical properties are different. Although the atomic mass is the same, the atomic number is not. This is due to the fact that the difference in the number of nucleons is offset by an increase in the number of neutrons.
On the other hand, we can say that the isotopes are atoms that have different numbers of neutrons but the same number of protons. It is possible to deduce from the foregoing definitions of atomic mass and atomic number that isotopes are elements with the same atomic number but different mass numbers.
Isobars
Isobar is a chemically different element with physical properties that are comparable. As a result, isobars can be defined as elements with various atomic numbers but the same mass number. They also have varied chemical properties due to the difference in electron counts. Because an additional number of neutrons compensates for the number of nucleons, an isobar has the same atomic mass but a different atomic number.
It is known that atomic mass is the sum of the number of protons and neutrons. As a result, the atomic mass of an atom is equal to the number of nucleons contained in the nucleus. And it will have the same number of nucleons as the previous one.
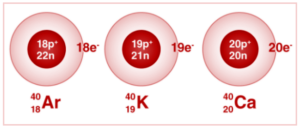
The number of nucleons, or the sum of protons and neutrons in isobars, will remain constant despite variations in the number of protons and neutrons. Isobars always have different atomic structures due to the difference in atomic numbers. The disparity in the number of nucleons is compensated for by the number of neutrons. As a result, they are always distinct chemical elements with the same atomic mass. As a result, isobar exhibits distinct chemical characteristics.
Examples of Isobars
Potassium, Argon, and calcium all have the same mass number of 40 atoms.
19 K 40, 18 Ar 40, 20 Ca 40
The isobars are represented by the digits 19, 18, and 20 as subscripts, which are the atomic numbers of the three elements, respectively. Because their atomic numbers differ, so do their chemical characteristics.
Isotopes
We observe that the superscript number present at the left of the element designation indicates the number of protons plus neutrons in the isotope, which means that the masses of different isotopes of an element vary since they have variable numbers of neutrons but the same number of protons and electrons.
The atomic number is the total number of protons in an atom’s nucleus and the number of electrons in a neutral (non-ionized) atom’s nucleus. Each atomic number identifies a specific element, but not an isotope; the number of neutrons in a given element’s atom can vary significantly. And, the amount of nucleons (both protons and neutrons) in an atom’s nucleus determines its mass number, thus each isotope of a given element has a distinct mass number.
Frederick Soddy, a radiochemist, was the one who discovered the isotopes. And, despite the periodic chart only allowing for 11 elements between lead and uranium included, investigations of radioactive decay chains showed around 40 distinct species referred to as radioelements (i.e. radioactive elements) between uranium and lead in 1913.
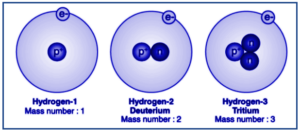
Examples of Isotope
Isotopes of hydrogen and carbon are common examples. If we consider hydrogen, there are three stable isotopes: protium, deuterium, and tritium. Apparently, protium, deuterium, and tritium have almost the same number of protons but different numbers of neutrons: protium has zero, deuterium has one, and tritium has two.
Whenever it comes to carbon, there are three isotopes: Carbon-12, Carbon-13, and Carbon-14. Here, the atomic masses of the given isotopes are 12, 13, and 14. Carbon-12 is a stable isotope in this situation, whereas carbon-14 is primarily a radioactive isotope.
And the other known isotopes include: Tin has 22 isotopes, Zinc has 21 known isotopes, Neon is a mixture of 3 isotopes, natural xenon is a mixture of 9 stable isotopes, and Nickel has 14 known isotopes.
Difference Between Isotopes and Isobars
| Isobars | Isotopes |
| They can be chemical elements with the same mass. | They can be the different atomic structures of the same element. |
| The physical properties are often similar. | There are various physical properties in general. |
| They possess the same atomic masses. | It possess different atomic masses |
| They have similar chemical elements. | It have similar chemical elements, yet they are packaged differently. |
| They possess different atomic numbers. | It possess the same atomic numbers. |
Also read: Important Topic Of Physics: Isotones
FAQs
Question 1: What is the importance of isobar?
Answer: Even if you’re at the same altitude, atmospheric pressure varies depending on where you are. Isobars are reference lines that run alongside a path and have the same pressure all the way along. The user can receive a realistic approximation at any map point using a series of lines, each denoting a site where the pressures have a comparable set value.
Question 2: Why do elements have isobars and isotopes?
Answer 2: t has distinct isotopes due to the existence of a varied number of neutrons in atoms of the same element. Due to the identical mass number (s) of neutrons and protons, various elements might have distinct isobars.
Question 3: Why are Isotopes dangerous?
Answer 3: Inhaling radioisotopes can cause DNA damage. Radioactive isotopes in the stomach can irradiate for a long time. Extremely high doses can cause sterility or mutations in the body. Radiation can also cause skin cancer and burns.





