Table of Contents
Introduction
The lead-acid battery is a type of rechargeable battery that was invented by French physicist Gaston Planté in 1859. It is the first rechargeable battery ever invented. Lead-acid batteries have a low energy density when compared to modern rechargeable batteries. Despite this, the cells have a relatively high power-to-weight ratio due to their ability to supply high surge currents. These characteristics, combined with their low cost, make them appealing for use in motor vehicles to supply the high current required by starter motors. Lead-acid batteries are widely used because they are less expensive than newer technologies, even when surge current is not a concern and other designs could provide higher energy densities. In 1999, lead-acid battery sales accounted for 40–50% of the total value of batteries sold worldwide (excluding China and Russia), equating to a $15 billion manufacturing market.
Large-format lead-acid designs are commonly used for backup power supplies in cell phone towers, high-availability settings such as hospitals, and stand-alone power systems. Modified versions of the standard cell may be used for these roles to improve storage times and reduce maintenance requirements.
Gel-cell and absorbed glass-mat batteries, collectively known as VRLA (valve-regulated lead-acid) batteries, are commonly used in these roles.
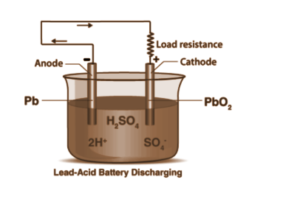
The chemical energy of the battery is stored in the potential difference between pure lead on the negative side and PbO2 on the positive side, plus the aqueous sulfuric acid when the battery is charged. The electrical energy released by a discharging lead–acid battery can be attributed to the energy released when the strong chemical bonds of water (H2O) molecules are formed from acid H+ ions and PbO2 ions. During charging, the battery, on the other hand, acts as a water-splitting device.
Overview
The electrodes, also known as grids, are made of a lead alloy frame to improve the grid’s strength and hardness while supporting the so-called active mass. This active mass is created by combining ultrafine Pb powder, lead oxide (PbO or Pb304), and diluted H2S04. Following drying, the mass composition consists primarily of lead sulfate and unreacted lead oxides. The final electrodes are formed by distributing the mass over the grids and charging them with H2SO4 electrolysis. This results in the formation of Pb at the negative electrode and PbC>2 at the positive electrode, as the concentration of sulphuric acid rises. The electrochemical reactions are reversed during discharge, producing PbSC>4 at both electrodes while the acid concentration decreases.
Components of a Battery
The following are the primary components of a lead/acid accumulator:
- alloyed metallic Pb with Sb and/or Ca;
- an electrolyte with diluted H2SO4;
- plastic materials to construct the separator and case
When car batteries are discharged for extended periods of time, the lead sulfate buildup can become extremely difficult to remove. This is why lead-acid batteries should be charged as soon as possible (to prevent the building up of lead sulfate). Typically, lead batteries are charged by providing an external current source. A plug is inserted and connected to the lead-acid battery, and the chemical reaction reverses. The charging process may become inefficient if the sulphuric acid in the battery (or another component of the battery) has decomposed. As a result, it’s a good idea to check the battery on a regular basis.
Lead-acid accumulator
Some electrochemical cells are rechargeable, which means that the electrode reactions are reversible and the process can be repeated multiple times. Electricity can be stored in such cells. The familiar lead-acid accumulator (‘car battery’) found in most combustion-engined vehicles is the most common type of heavy-duty rechargeable cell. A simple lead-acid cell is built and charged for various lengths of time using lead strips and a dilute sulfuric acid electrolyte. The cell is then discharged through a light bulb, and the duration of the bulb’s illumination is measured. A graph plotting this time versus charging time demonstrates the relationship between the electrical energy put into the cell and the energy released.
The storage battery, also known as the secondary battery, is a type of battery in which electrical energy is stored as chemical energy and then converted to electrical energy when needed. Charging a battery is the process of converting electrical energy into chemical energy by using an external electrical source. Whereas secondary battery discharging refers to the conversion of chemical energy into electrical energy for the purpose of supplying an external load. When a battery is charged, current flows through it, causing chemical changes inside the battery. During their formation, these chemical changes absorb energy.
The electrodes of an accumulator are made of lead, and the electrolyte is dilute sulphuric acid. The electrodes are typically made of a lead alloy that contains 7–12 percent antimony (for increased hardness and corrosion resistance) and a trace of tin (for better casting properties). After inserting the electrodes into the electrolyte, a ‘forming’ current is passed through the cell to convert the PbO on the negative plate into a sponge of finely divided lead. PbO is converted to lead(IV) oxide on the positive plate (PbO2). The overall reaction equation during discharge is,
PbO2+2H2SO4+Pb → 2PbSO4+2H2O.
What is a lead accumulator?
A lead accumulator is a secondary cell because electrical energy is not generated within it but is instead stored there from an external source. It is a reversible cell because cell reactions are reversed when an external e.m.f. just greater than this cell’s e.m.f. is applied. Thus, the net cell reaction in this cell can be reversed by applying an external opposing e.m.f. greater than the cell e.m.f. This cell is capable of storing electrical energy from an external source (charging) and supplying it during discharging. Chemical energy is used to store the energy. As a result, this cell is a storage cell, accumulator, or storage battery. Its voltage is determined by the strength of a sulphuric acid solution rather than the size of the electrodes or the size of the cell.
Lead accumulator cell
The lead accumulator is a secondary cell because the electrical energy is not generated inside the cell but is stored prior to being supplied by an external source. It is a reversible cell because the cell reactions are reversed when an external emf is applied that is greater than the emf applied to this cell.
FAQ’s
What exactly is a lead-acid battery?
In the fully charged state, the negative plate is made of lead and the positive plate is made of lead dioxide. The electrolyte is concentrated sulphuric acid, which retains the majority of the chemical energy.
How is a lead-acid battery manufactured?
The lead battery is made of two chemically dissimilar lead-based plates immersed in a sulphuric acid solution using lead alloy ingots and lead oxide.
What is the operation of a lead accumulator?
A lead accumulator is a secondary cell because electrical energy is not generated within the cell but is instead stored in it from an external source. This cell is capable of storing electrical energy from an external source (charging) and supplying it during discharging.








