Table of Contents
What is Pauli Exclusion Principle and what is its significance? In this article, we shall learn why it is critical to understand Pauli Exclusion Principle when studying electrons. Before we delve into the topic, let us try to get an overview of the electrons and atoms first.
Overview
Electrons are much smaller than protons and neutrons, weighing over 1,800 times less than either. The electron configuration of an atom is the orbital description of the electron locations in a typical atom. Chemists can anticipate an atom’s attributes, such as stability, boiling temperature, and conductivity, using the electron configuration and physical principles. It is the method or distribution of electrons in an atom’s orbitals. An atom is made up of subatomic particles such as electrons, protons, and neutrons, with only the number of electrons being taken into account for electronic arrangement. Electrons are supplied in such a way that a high constant configuration is achieved.
The electron shells are denoted by the letters K, L, M, N, O, P, and Q, or by the numbers 1, 2, 3, 4, 5, 6, and 7, counting from the innermost to the outermost shell. Every shell is made up of one or more subshells, which are made up of atomic orbitals and are referred to as subshells.
The quantum property of electrons is electron spin. It is an example of angular momentum. This angular momentum’s magnitude value is fixed. Spin, like charge and rest mass, is a fundamental, unchanging feature of the electron. The spin angular momentum associated with electron spin is distinct from the orbital angular momentum associated with electrons traveling around the nucleus.
Pauli Exclusion Principle
In chemistry, Pauli’s Exclusion Principle, along with Aufbau’s Principle and Hund’s Rule, is one of the most significant principles. It is critical for students to understand, especially when studying electrons. Pauli’s Exclusion Principle essentially helps us comprehend the electron configurations in atoms and molecules and also provides an explanation for the periodic table’s classification of elements.
No two electrons in an atom may have the same set of four quantum numbers, according to Pauli’s Exclusion principle. Only two electrons can coexist in the same orbital, and they must have opposing spins.
Pauli’s Exclusion principle has two rules:
- Two electrons occupy the same orbital.
- Two electrons in the same orbital have opposing spins or are antiparallel.
Pauli’s Exclusion principle applies not just to electrons but also to other particles such as half-integer spin. Wolfgang Pauli developed the principle in 1925. It is unimportant for particles with integer spins, such as bosons, which have symmetric wave functions. Furthermore, unlike fermions, bosons can share or have the same quantum states
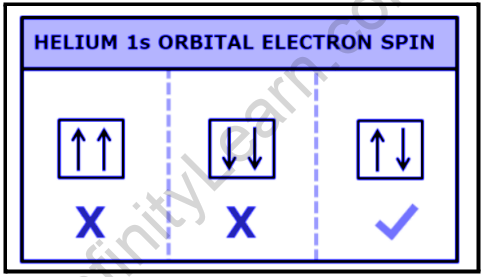
As an example of Pauli’s Exclusion principle, consider a neutral helium atom. The atom is connected to two electrons, which occupy the outermost shell with opposing signs.
There are two electrons in the 1s subshell: n=1,l=0,ml=0.
If we construct a diagram of a helium atom, we can see that it has one electron up and one electron down.
Significance of Pauli’s Exclusion Principle:
- This certain principle can be used to explain a wide range of physical events.
- It aids in demonstrating how the elements work together to generate chemical bonds.
- This rule can also be used to describe a periodic table.
Hund’s Rule or Hund’s Rule of Maximum Multiplicity
The Pauli exclusion principle describes how electrons occupy a single orbital. Many subshells, however, include more than one orbital. The two portions of Hund’s rule describe how to fill a subshell containing many orbitals.
Hund’s rule asserts that electron pairing in orbitals belonging to the same subshell does not occur until each orbital in that subshell possesses one electron. For example, if there are three p orbitals, five d orbitals, and seven f orbitals, electron pairing begins with the p, d, and f orbitals with the fourth, sixth, and eighth electrons.
There is some level in each orbital that contains two electrons; when the electron is filled in these orbitals, the sublevel is initially filled with one electron in the same spin. After finishing all of the sub-levels, the electron field in each level doubles with the opposite sign. For the p orbital, which has three subshells, three electrons will be a field in the same spin, and the remaining three electrons will be a field in the opposite spin of the same sublevels, one by one.
When electrons are allowed to orbitals, an electron strives to fill all of the orbitals with equivalent energy (also known as degenerate orbitals) before merging with another electron in a half-filled orbital. Atoms in ground states have as many unpaired electrons as possible.
Consider how electrons behave in the same way that the same poles on an attraction would if they came into contact with each other; as negatively charged electrons fill orbitals, they initially strive to go as far away from each other as possible before having to match up.
Additionally, quantum-mechanical simulations have revealed that only electrons in filled orbitals are tiny, adequately screened, or insulated from the nucleus.
Hund’s rule is significant because it dictates the sequence in which electrons are filled in a set of orbitals. The orbital filling is a bonded diagram that shows the placement of electrons in distinct orbitals.
FAQs
Question 1: What is the difference between Hund’s rule and Pauli’s Exclusion Principle?
Answer 1: Hund’s rule predicts the presence of two or more degenerate states and how electrons can occupy them. Whereas Pauli’s exclusion principle indicates which types of electrons can be filled together.
Question 2: Why do we refer to Hund’s rule as the rule of maximum multiplicity?
Answer 2: Hund’s rule is known as the rule of maximum multiplicity because, among all potential electronic configurations, only the one with the highest total spin value is accurate.
Question 3: Why is Hund’s rule not applicable for all elements?
Answer 3: If Hund’s rule is ignored, the number of singly occupied orbitals or unpaired electrons will steadily decrease. If Hund’s rule is obeyed, the total number of unpaired electrons is 5. As a result, if this rule is broken, there will be only one unpaired electron. Hund’s rule does not apply to the NO molecule.
Question 4: What configurations are in violation of Hund’s principle?
Answer 4: Before you deposit two electrons in the same orbital, each orbital with the same energy must have at least one electron with the same spin.

