Table of Contents
Introduction:
Hydrogen (H), the simplest member of the chemical element family, is a colorless, odorless, tasteless, combustible gaseous substance. Henry Cavendish, an English physicist, discovered hydrogen in 1766. He termed it ‘inflammable air.’ In 1783, French scientist Antoine Lavoisier named its hydrogen.
It may be found as dihydrogen in its molecular form. It accounts for 70% of the entire mass of the cosmos. It is the most important component of the solar system. Large planets like Jupiter and Saturn are mostly made up of hydrogen. It makes up 15.4 % of the earth’s crust and seas when combined.
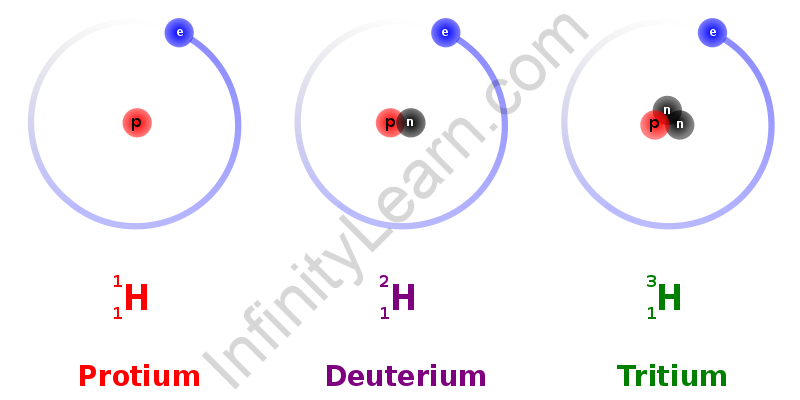
Hydrogen is found as dihydrogen in its molecular form. Because of its dual nature, hydrogen has a wide range of applications, making it a prominent element in the chemical industry. There are three hydrogen isotopes. The hydrogen molecule creates the H-H bond, which has the greatest bond enthalpy between two atoms of an element. Protium is the most frequent isotope of hydrogen found in nature, out of all three isotopes.
Overview:
Because of its similarities to alkali metals and halogens, hydrogen is a one-of-a-kind element on the periodic table.
It is the first element in the periodic table. It shares properties with alkali metals and halogens. Despite the fact that scientists have discovered hydrogen in its metallic form in recent studies and tests. It, like other alkali metals, contains one electron in its outermost shell and forms the monovalent ion, but in terms of ionization energy, it is similar to halogens.
There are three hydrogen isotopes: protium H11, deuterium H12, or D, and tritiumH13 or T. Because of the varying quantities of neutrons in them, the isotopes differ. Isotopes are elements that have the same atomic number but a different mass number. Protium is the most frequent isotope of hydrogen found in nature, out of all three isotopes.
Properties of Hydrogen:
Physical Properties of Dihydrogen:
- It is a colorless, odorless, and tasteless gas.
- It is insoluble in water.
- It is extremely flammable.
- It is less dense than air.
- It has a melting point of 13.96 K.
- It has a boiling point of 20.39 K.
- It has a density of 0.09 g/L.
- It has a fusion enthalpy of 0.117 kJ/mol.
Chemical Properties of Dihydrogen:
The bond disassociation enthalpy is a major determinant of chemical characteristics. The hydrogen molecule creates the H-H bond, which has the greatest bond enthalpy between two atoms of an element. Because of its high bond enthalpy, hydrogen is inert at ambient temperature and must be produced at very high temperatures in an electric arc or under UV radiations. Because hydrogen has an electrical configuration of 1s1, it can react by losing an electron and creating an H+ ion, or by gaining an electron and forming an H– ion. Hydrogen creates a covalent bond by sharing electrons with other elements.
Dihydrogen is very stable and only dissociates into hydrogen atoms when heated beyond 2000 K, H2H+H. It has a very high bond dissociation energy, H=435.9 KJ/mol.
It is not highly reactive due to its high bond dissociation energy. It does, however, mix with a wide range of elements and compounds.
It depicts the following reactions:
- Reactions with metals: At high temperatures, it reacts with numerous metals to form the corresponding hydrides.
H2(g)+2M (g)2MH(s) ; where M is an alkali metal
- Reaction with halogens: When it interacts with halogens, it produces hydrogen halides.
H2(g)+X2(g)2HX(g) (X=F,Cl,Br,I)
- Reaction with dioxygen: Water is formed as a result of its reaction with dioxygen. The process is very exothermic.
2H2(g)+O2(g)2H2O(l)
- Reaction with dinitrogen: It yields ammonia when it reacts with dinitrogen.
The Haber process is used to produce ammonia in this manner.
3H2(g)+N2(g)2NH3(g)
- Reaction with Metal ions and metal oxides: It converts certain metal ions in an aqueous solution and metal oxides (less active than iron) into equivalent metals.
H2(g)+Pd2+(aq)Pd(s)+2H+(aq)
- Reactions with organic compounds: In the presence of catalysts, it interacts with various organic molecules to produce valuable hydrogenated products of economic value.
For instance, nickel-catalyzed hydrogenation of vegetable oils yields edible lipids (margarine and vanaspati ghee).
H2+CO+RCH=CHRCH2CH2CHO
Uses of Dihydrogen:
Hydrogen has a wide range of applications because of its dual nature;
- It is employed in the synthesis of metal hydrides.
- It is utilized in the production of vanaspati fats.
- It is involved in the manufacturing of nitrogenous fertilizers and the synthesis of ammonia.
- By using metallurgical procedures, hydrogen can convert several metal oxides to metals.
- In many space research endeavors, hydrogen is employed as rocket fuel.
- Hydrogen fuel cells are being used in the automobile sector to test hydrogen fuel.
- Torches made of atomic hydrogen and oxy-hydrogen are used for cutting and welding.
- It acts as a reducing agent.
- It is used in the Commercial nitrogen fixation from the air in the Haber ammonia process.
- It’s used to fill balloons (hydrogen gas much lighter than air; however it ignites easily)
- It is also employed as a tracer isotope and as a radioactive agent in the production of bright paints.
- It is utilized in the production of bulk organic compounds, specifically methanol.
Also read: Important Topic of Chemistry: Enthalpy
FAQs:
Q. What do you mean by the term Hydrogenation?
Ans: Hydrogenation is a chemical reaction that occurs between molecular hydrogen and another element or molecule, usually with the help of a catalyst, which is usually a metal such as nickel, palladium, or platinum or its oxides.
Q. Why do humans require hydrogen?
Ans: Hydrogen is a vital component of water. Water keeps the body’s cells hydrated and aids in the removal of toxins. Hydrogen allows the body to generate energy.
Q. Is hydrogen toxic?
Ans: Although hydrogen is not harmful, if one breathes it in its pure form, one might die from asphyxiation due to oxygen deprivation. Inhaling hydrogen in low amounts can produce nausea, headache, skin and eye irritation, and convulsions. However, inhaling a high quantity of hydrogen might result in asphyxiation. Liquid hydrogen is very chilly due to its high compression. It can also cause serious frostbite if it escapes from the vehicle tank and comes into touch with the skin.
Q. What is syn-gas?
Ans: Synthesis gas, sometimes known as “syngas,” is a combination of carbon monoxide and hydrogen that is utilized in the production of methanol and other hydrocarbons.