Table of Contents
The concept of entropy as a physical property of a thermodynamic system is established by the second law of thermodynamics. It can be used to predict whether processes are prohibited despite obeying the requirement of energy conservation as expressed in the first law of thermodynamics, and it provides necessary criteria for spontaneous processes. The second law can be expressed as the observation that the entropy of isolated systems left to spontaneous evolution cannot decrease because they always arrive at a state of thermodynamic equilibrium with the highest entropy at the given internal energy. The irreversibility of natural processes is explained by an increase in the combined entropy of the system and its surroundings, which is often referred to in the concept of the arrow of time.
Historically, the second law was an empirical discovery that was accepted as a thermodynamic theory axiom. Statistical mechanics explains the law at a microscopic level in terms of probability distributions of the states of large assemblies of atoms or molecules. Many different ways have been proposed to express the second law. Carnot’s theorem, credited to the French scientist Sadi Carnot, who demonstrated in 1824 that the efficiency of converting heat to work in a heat engine has an upper limit, was its first formulation, which preceded the proper definition of entropy and was based on caloric theory.
The first rigorous definition of the second law based on the concept of entropy came in the 1850s from German scientist Rudolph Clausius, who stated that heat can never pass from a colder to a warmer body without some other change occurring concurrently. The concept of thermodynamic temperature can also be defined using the second law of thermodynamics, but this is usually delegated to the zeroth law of thermodynamics.
Overview
The second law of thermodynamics limits the direction of heat transfer and the efficiency of heat engines. According to the first law of thermodynamics, the energy of the universe remains constant; while energy can be exchanged between system and surroundings, it cannot be created or destroyed. While the first law of thermodynamics provides information about the quantity of energy transferred as a process, it does not provide information about the direction of energy transfer or the quality of the energy. The first law does not explain how a metallic bar of uniform temperature can spontaneously become warmer at one end and cooler at the other. All the law can say is that if the process occurs, there will always be an energy balance. The criterion for the feasibility of any process is provided by the second law of thermodynamics. A process cannot occur unless both the first and second laws of thermodynamics are satisfied.
According to the second law of thermodynamics, any spontaneously occurring process will always result in an increase in the entropy (S) of the universe. In layman’s terms, the law states that the entropy of an isolated system will never decrease over time. However, when a system is in thermodynamic equilibrium or is undergoing a reversible process, the total entropy of the system and its surroundings remains constant. The second law is also referred to as the Law of Increasing Entropy.
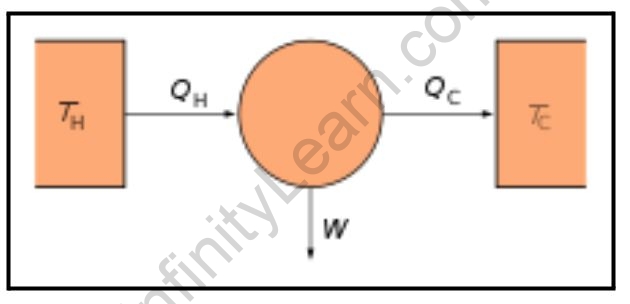
The second law states unequivocally that it is impossible to convert heat energy to mechanical energy with 100% efficiency. When we look at a piston in an engine, for example, the gas is heated to increase its pressure and drive a piston. Even as the piston moves, there is always some leftover heat in the gas that cannot be used for any other purpose. Heat is squandered and must be discarded. In this case, waste heat is discarded by exhausting the used fuel and air mixture to the atmosphere, or in the case of a car engine, waste heat is discarded by transferring it to a heat sink. Furthermore, heat generated by friction that is generally ineffective should be removed from the system.
Second law of thermodynamics
The total entropy of an isolated system always increases over time, or remains constant in ideal cases where the system is in a steady-state or undergoing a reversible process, according to the second law of thermodynamics. The irreversibility of natural processes is explained by the increase in entropy.
Second law of thermodynamics equation
The second law of thermodynamics is represented mathematically as;
∆Suniv >0
where ∆Suniv is the change in the universe’s entropy.
Entropy is a measure of a system’s randomness, or it is a measure of energy or chaos within an isolated system. It can be thought of as a quantitative index describing the quality of energy. Meanwhile, there are only a few factors that increase the entropy of a closed system. To begin, while the mass remains constant in a closed system, there is an exchange of heat with the surroundings. This change in heat content causes a disturbance in the system, increasing the system’s entropy. Second, internal changes in the system’s molecule movements may occur. This causes disturbances, which in turn cause irreversibilities within the system, increasing its entropy.
There are two statements about the second law of thermodynamics:
Kelvin-Planck Statement:
A heat engine cannot produce a network in a complete cycle if it only exchanges heat with bodies at a single fixed temperature.
Clausius’s Statement:
It is impossible to build a device that can transfer heat from a colder body to a warmer one without consuming any work. Furthermore, energy will not spontaneously flow from a low-temperature object to a higher-temperature object. It is critical to understand that we are discussing the net transfer of energy. Energy can be transferred from a cold object to a hot object through the transfer of energetic particles or electromagnetic radiation. In any spontaneous process, however, the net transfer will occur from the hot object to the cold object. And work is required to transfer the net energy to the hot object.
Also read: Important Topic of Chemistry: Hess’s Law
FAQs
Q. What is the second law of thermodynamics?
Ans: The total entropy of an isolated system (the thermal energy per unit temperature that is unavailable for doing useful work) can never decrease, according to the second law of thermodynamics.
Q. What is the Clausius statement of thermodynamics’ second law?
Ans: According to Clausius’ interpretation of the second law of thermodynamics, “It is impossible to construct a device that operates in a cycle and produces no effect other than the transfer of heat from a cooler body to a hotter body.”





