Table of Contents
Introduction:
Sodium bicarbonate, also known as baking soda or bicarbonate soda (IUPAC name: sodium hydrogen carbonate), is a chemical compound with the formula NaHCO3. It is a salt made up of sodium cation (Na+) and bicarbonate anion HCO3. Sodium bicarbonate is a white crystalline substance that is commonly found in the form of a fine powder. It has a slightly salty, alkaline flavour similar to washing soda (sodium carbonate). Nahcolite is the natural mineral form.
It is a constituent of the mineral natron and can be found dissolved in a variety of mineral springs. Because it has been around for a long time and is widely used, the salt is known by many different names, including baking soda, bread soda, cooking soda, and bicarbonate of soda, and can be found in stores near baking powder.
In the United States, baking soda is more commonly used, whereas bicarbonate of soda is more commonly used in Australia, the United Kingdom, and Ireland. It is also known as Natron in many northern/central European countries. Common colloquial forms include sodium bicarb, bicarb soda, bicarbonate, and bicarb. Sodium bicarbonate, the monosodium salt of carbonic acid, possesses alkalinizing and electrolyte replacement capabilities.
The sodium and bicarbonate ions separate from sodium bicarbonate. Ion formation raises blood pH by increasing plasma bicarbonate and buffering excess hydrogen ion concentration. Sodium bicarbonate is a white, crystalline powder that is commonly used as a pH buffer, electrolyte replenisher, systemic alkalizer, and in topical cleansing solutions.
Overview: Sodium Hydrogen Carbonate
This article explains the Sodium Hydrogen Carbonate formula, also known as the Sodium Bicarbonate formula or Baking Soda formula. It is a weakly basic inorganic compound made up of cations (Sodium) and anions (Bicarbonate). NaHCO3 is the chemical or molecular formula for Sodium Hydrogen Carbonate. It has a crystalline solid white colour and no odour. It has a density of 2.2 g/mL in its crystalline form and a density of 1.2 g/mL in its powder form. It dissolves easily in water. The Solvay process produces it by reacting sodium chloride with ammonia, water, and carbon dioxide to produce NaHCO3 and ammonium chloride salt.
Sodium Hydrogen Carbonate Formula Structure
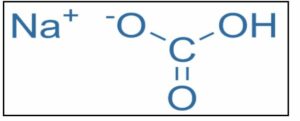
The crystalline structure of sodium bicarbonate or sodium hydrogen carbonate is monoclinic. In 1791, Nicolas Leblanc, a French chemist, invented sodium carbonate. Austin Church and John Dwight, New York bakers, established the first factory to produce baking soda in 1846. It is a white solid crystalline chemical compound that is usually in the form of powder. This salt is made up of sodium and bicarbonate ions. It has the molecular formula NaHCO3. It is a shaky foundation. Baking soda is a type of baking soda that is commonly used in cooking. The PH value is approximately 8.31.
Preparation and Properties sodium hydrogen carbonate
The Solvay procedure is used to manufacture sodium bicarbonate and sodium carbonate in the industrial world. This process uses carbon dioxide, water, ammonia, and concentrated brine solution as basic materials. This process is favoured because it is less expensive and uses fewer raw materials to produce the chemicals necessary. Baking soda and sodium carbonate are created using the following chemical reaction:
CO2+H2O+NH3+NaCl→NaHCO3+NH4Cl 2NaHCO3→Na2CO3+CO2+H2O
The produced carbon dioxide is recycled to produce NaHCO3
Properties
- It is non-combustible.
- Unlike other types of dust, powder dust isn’t as explosive.
- It has a melting point of 50°C and is an odorless white crystalline solid.
- It is fundamental in nature.
Use of sodium hydrogen carbonate
- It is used as a pesticide to kill cockroaches and to control fungal growth.
- It is used as a disinfectant to protect the armpits from odour and irritation.
- It is used in cooking, particularly to bake food.
- It is used in medicine to prevent the side effects of chemotherapy by being injected intravenously.
- Because of its antibacterial properties, it is used to clean kitchen items.
- It is used to keep your teeth and mouth clean.
Sodium hydrogen carbonate soluble in water
Humans have been using sodium bicarbonate for thousands of years. In ancient Egyptian documents, sodium bicarbonate and sodium chloride solution are mentioned in the mummification of the dead. People all over the world have used sodium bicarbonate as a leavening agent in baking for centuries. A leavening agent is an ingredient that causes dough or batter to rise. This effect is caused by sodium bicarbonate, which, when heated or dissolved in water, decomposes to produce carbon dioxide (CO2) gas:
2NaHCO3→Na2CO3+CO2+H2O
Sodium bicarbonate is an excellent leavening agent because all of the compounds present in this reaction are safe for human consumption.
Also read: IUPAC Nomenclature of Organic Compounds







