Table of Contents
Intoduction:
Carbohydrates are a class of carbonyl compounds (aldehydes or ketones) that contain several hydroxyl groups and are found in nature. It could also include derivatives that produce similar compounds when hydrolyzed. They are also known as “saccharides” and are the most abundant organic molecules in nature. Sugars are sweet-tasting carbohydrates that are soluble in water.
Sugars make up a portion of that carbohydrate. These provide a ready source of fuel for the potato and the person eating it. Fiber, which includes cellulose polymers that give structure to the potato’s cell walls, makes up a little more of the carbohydrate in the potato.
The majority of carbohydrate is in the form of starch, which is made up of long chains of linked glucose molecules and is used as a storage fuel. Enzymes in your digestive tract break down long glucose chains into smaller sugars that your cells can use when you eat French fries, potato chips, or a baked potato with all the fixings.Carbohydrates are biological molecules that are composed of carbon, hydrogen, and oxygen in a ratio of one carbon atom to one water molecule.
Carbohydrate Properties:
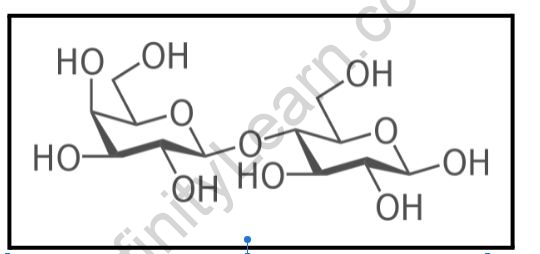
Carbohydrate Physical Properties:
- Stereoisomerism refers to compounds that have the same structural formula but differ in their spatial configuration. When it comes to the penultimate carbon atom, there are two isomers of glucose. These are the two sugars: D-glucose and L-glucose.
- Optical Activity – The rotation of plane-polarized light that produces (+) and (-) glucose.
- Diastereoisomers are configurational changes in glucose involving C2, C3, or C4. Mannose and galactose are two examples.
- Anomerism is the spatial configuration of the first carbon atom in aldoses and the second carbon atom in ketoses with respect to the first carbon atom.
Carbohydrate Chemical Properties:
- Sugars react with an excess of phenylhydrazine to form osazone, which is a carbohydrate derivative. Glucosazone, for example. In terms of the penultimate carbon atom, there are two isomers of glucose. The two sugars are D-glucose and L-glucose.
- Benedict’s challenge: When reducing sugars are heated in the presence of an alkali, they are converted to enediols, which are powerful reducing species. When Benedict’s reagent solution and reducing sugars are heated together, they turn an orange-red/brick red colour.
- Oxidation: If the carbonyl groups in monosaccharides oxidize to form carboxylic acids, they are referred to as reducing sugars. D-glucose is oxidized to D-gluconic acid in Benedict’s test, making glucose a reducing sugar.
- Reduction of alcoholic beverages: Sodium borohydride, NaBH4, or catalytic hydrogenation (H2, Ni, EtOH/H2O) can reduce the C=O groups in open-chain carbohydrates to alcohols.”Alditols” is the name given to the products.
Structure of carbohydrates:
- Carbon, hydrogen, and oxygen make up the molecules of carbohydrates.
- The empirical structure of carbohydrates is (CH2O)n.
- They are organic compounds with multiple hydroxyl groups coming off the carbon chain that are organized as aldehydes or ketones.
- The building blocks of all carbohydrates are monosaccharides, or simple sugars.
- A monosaccharide is defined as a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose).
Carbohydrates are structurally represented in one of three ways:
- Structure with an open chain.
- The long straight-chain form of carbohydrates is known as the open-chain structure.
- The structure is hemiacetal.
- The 1st carbon of glucose condenses with the -OH group of the 5th carbon to form a ring structure in the hemi-acetal structure.
- The structure of Haworth.
- The presence of the pyranose ring structure is called the Haworth structure.
Classification of carbohydrates:
Monosaccharides, polymers, oligosaccharides, and polysaccharides are all examples of simple carbohydrates.
Monosaccharides
- Because they cannot be further hydrolyzed, this group of carbohydrates is often referred to as simple sugars.
- Colorless, crystalline solids that are water-soluble but not non-polar solvent-soluble.
- These are substances that contain a free aldehyde or ketone group.
- Cn(H2O)nor CnH2nOn is the general formula.
- They are classified by the number of carbon atoms they contain as well as the functional group they contain.
- Trioses, tetroses, pentoses, hexoses, heptoses, and other monosaccharides with 3, 4, 5, 6, 7,… carbons are known as trioses, tetroses, pentoses, hexoses, heptoses, and aldoses or ketoses, depending on whether they contain aldehyde or ketone group.
- Glucose, Fructose, Erythrulose, and Ribulose are some examples.
Oligosaccharides
- When oligosaccharides are hydrolyzed, they produce 2 to 10 molecules of the same or different monosaccharides.
- A glycosidic linkage connects the monosaccharide units.
- Depending on the number of monosaccharide units, it is classified as a disaccharide, trisaccharide, tetrasaccharide, or tetrasaccharide.
- A disaccharide is an oligosaccharide that yields two molecules of monosaccharides when hydrolyzed, while trisaccharides and tetrasaccharides yield three or four monosaccharides, and so on.
- The general formula for disaccharides is Cn(H2O)n-1, while the formula for trisaccharides is Cn(H2O)n-2, and so on.
- Examples of disaccharides include sucrose, lactose, maltose, and other sugars.
- Trisaccharides are divided into two types: Raffinose and Rabinose.
Polysaccharides
- They’re also known as “glycans.”
- Polysaccharides are sugar molecules that contain more than 10 monosaccharide units and can be hundreds of sugar units long.
- When hydrolyzed, they produce more than 10 monosaccharide molecules.
- Polysaccharides differ from one another in terms of the identity of their recurring monosaccharide units, chain length, bond linking unit types, and branching degree.
- They are primarily concerned with two key functions: structural functions and energy storage.
- They’re further divided into groups based on the types of molecules produced during hydrolysis.
- They can be homopolysaccharides, meaning they contain monosaccharides of the same type, or heteropolysaccharides, meaning they contain monosaccharides of different types.
- Starch, glycogen, cellulose, and pectin are examples of homopolysaccharides.
Function of carbohydrates:
- Carbohydrates are a type of molecule that can also be found in both animal and plant tissues. Carbohydrates from the skeletal structures of plants and arthropods serve as food reserves in plants and animals. They are important sources of energy for a variety of metabolic activities, and the energy is obtained through oxidation.
- Carbohydrates are used by living organisms to power cellular reactions. They are the most abundant dietary source of energy for all living things (4kcal/gram).
- Carbohydrates, in addition to being the primary energy source in many animals, are also instant energy sources.
- Energy stores, fuels, and metabolic intermediates are all used. Glycogen is deposited by animals, whereas starch is stored by plants.
- Instead of proteins, stored carbohydrates are used as an energy source.
- They form structural and protective components in plant and microorganism cell walls. Animal cell walls (peptidoglycan or murein), bacterial cell walls (peptidoglycan or murein), and plant cell walls.
- Carbohydrates are used as a building block in the production of fats and proteins.
- Carbohydrates focus on providing energy to the brain and assist in nerve tissue regulation.
- Carbohydrates form surface antigens, receptor molecules, vitamins, and antibiotics when they combine with lipids and proteins.
- The structural frameworks of RNA and DNA, such as ribonucleic acid and deoxyribonucleic acid, are formed.
- Many proteins and lipids are linked to them. Interactions between cells and other elements in the cellular environment rely on linked carbohydrates for cell-to-cell communication.
- They are an important component of connective tissues in animals.
- Eating fiber-rich carbohydrates can help you avoid constipation.
- They also aid in the immune system’s modulation.
Importance of chapter for NEET and board exams:
Many young medical aspirants fail to balance their preparation for NEET and Class 12 board exams. The pressure to do well in Class 12 boards is enormous in a country like ours, where many people compete for a single mark. As a result, candidates preparing for both the NEET and the Class 12 board examinations must be able to juggle between the two effectively.
This article on ‘how to prepare for NEET and Class 12 board exams at the same time aims to assist candidates in preparing for both exams at the same time. The NEET 2022 exam is scheduled to take place in the first week of May, while the CBSE Class 12 board exam will begin in February/March.
Also read: NEET Exam Pattern 2022
FAQs
Question 1: What are carbohydrates and their function?
Answer 1: Carbohydrates are one of our diet’s three macronutrients, and their primary purpose is to provide energy to the body. They can be found in many different of forms, including sugars and dietary fiber, as well as in foods like whole grains, fruits, and vegetables.
Question 2: Do carbohydrates provide structure?
Answer 2: Carbohydrates are a class of macromolecules that provide structural support to plant cells, fungi, and all arthropods, such as lobsters, crabs, shrimp, insects, and spiders, as well as providing energy to the cell.
Question 3: What is the main role of carbohydrates?”
Answer 3: Carbohydrates provide energy to your body, which is one of their primary functions. The majority of the carbohydrates in your food are digested and broken down into glucose before entering your bloodstream.
Question 4: What are the benefits of carbohydrates?
Answer 4: Carbohydrates are your body’s primary energy source, helping to fuel your brain, kidneys, heart muscles, and central nervous system. Fiber, for example, is a carbohydrate that aids digestion, keeps blood cholesterol levels in check, and keeps you feeling full.







