Table of Contents
Structure of Triple Bond: What does triple bond mean? What is the structure of triple bond and what significance does it hold? We will try to answer to the above questions and others through this article.
In chemistry, a triple bond is a chemical bond between two atoms that involves six bonding electrons rather than the usual two in a covalent single bond. With a bond order of three, triple bonds are stronger than equivalent single or double bonds. Alkynes contain the most common triple bond, that between two carbon atoms. Cyanides and isocyanides are two other functional groups that contain a triple bond. Some diatomic molecules are also triple bonds, such as dinitrogen and carbon monoxide. The triple bond is represented in skeletal formulae by three parallel lines connecting the two connected atoms. A triple bond is formed between two carbon atoms when three pairs of electrons are shared between them. Alkynes are distinguished from other hydrocarbons by the presence of a triple bond between the carbon atoms. Acetylene’s triple-bonded carbon atoms are hybridized.
A head-on overlapping of the two hybridized sp orbitals results in a sigma bond between carbon atoms. The leftover orbitals of the carbon atoms overlap with each other in the 1s orbital of each hydrogen atom along the internuclear axis, resulting in the formation of one C-H sigma bond and two weaker pi bonds. These bonds form as a result of orbital overlapping. Each carbon atom only makes use of one of its three p-orbitals. As a result, a single electron occupies the two remaining p orbitals. Because it is the same as the other carbon atom, this allows electron pairing, resulting in the formation of two pi bonds.
The presence of a one sigma bond strengthens the carbon-carbon triple bond. Acetylene is a straight-line molecule with a carbon-carbon distance of 1.21Ao. Because sp hybridized carbon has more s character than sp2 hybridized carbon, the C-H distance in acetylene is 1.08 shorter than in alkenes.
Overview
Ethyne, also known as acetylene, is a chemical compound that has the formula C2H2. This compound is a hydrocarbon because its entire chemical composition consists of only hydrogen and carbon atoms. Many consider ethyne to be the most basic alkyne because it is made up of only two carbon atoms that are triply bonded to each other. Pure ethyne is notoriously unstable. As a result, it is not uncommon for ethyne to be used in a solution. Ethyne can be produced by partially combusting methane. This compound can also be made by hydrolyzing calcium carbide (a chemical compound with the formula CaC2, also known as calcium acetylide). The chemical equation for this reaction of calcium carbide and water is shown below. Because it contains a carbon-carbon triple bond, ethyne (or acetylene) is an unsaturated hydrocarbon.
H2O + CaC2 → C2H2 + Ca(OH)2
As a result of the hydrolysis of calcium carbide, the final products are calcium hydroxide and ethyne.
Ethyne formula
Ethyne, also known as acetylene, is the most basic triple-bonded 2 carbon organic compound. Acetylene’s IUPAC name is ethyne. The word ‘eth’ means ‘two,’ and ‘yne’ refers to a triple bond formed by two carbon atoms. They combine to form the IUPAC name and the meaning of ethyne. C2H2 is the formula for acetylene. The formula clearly indicates that it is a hydrocarbon because there are no elements other than carbon and hydrogen in the molecular structure. Acetylene is another name for ethyne. Ethyne is the most basic two carbon alkyne, with the formula HC≡CH and the molecular formula C2H2
The structural formula of ethyne
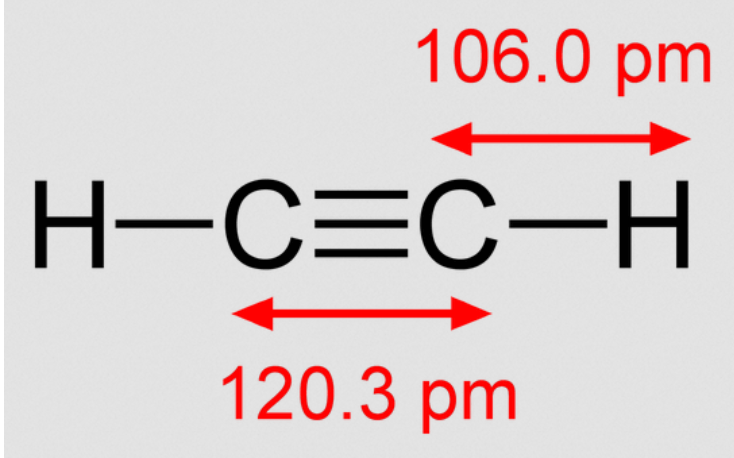
The ethyne molecule has a triple bond formed by two carbon atoms, each of which is singly bonded to one hydrogen atom. It is worth noting that all four atoms align in a straight line with bond angles of about 180o. It should also be noted that the carbon-hydrogen bond length in the ethyne molecule is approximately 106 picometres, whereas the carbon-carbon bond length in the molecule is approximately 120.3 picometres. The ethyne molecule’s structure is depicted below.
The electron dot structure of ethyne is another fascinating aspect to learn about on this concept page. You will need to study and practice the acetylene electron dot structure thoroughly in order to understand how electrons are shared in each bond in the molecule. Students will gain a better understanding of how the compound is formed by practicing the electron dot structure. The ethyne Lewis structure is another name for this. This concept is ideal for students who want to learn how organic bonds are formed by sharing electrons. You will also learn how to form a carbon-hydrogen bond and how electrons are shared between two atoms of different elements.
Structure of triple bond
The simplest molecule in the alkyne series is ethyne. In this case, each carbon atom is sp hybridized, resulting in the formation of two sp hybrid orbitals. These two sp hybrid orbitals are parallel and have a bond angle of 180 degrees.
The two unhybridized p orbitals are perpendicular to each other and to the hybridized orbitals’ axis. In the formation of a triple bond, one carbon atom’s sp hybridized orbital overlaps axially with the similar sp hybrid orbital of the other carbon atom to form a sigma bond. Each of one carbon’s two unhybridized orbitals overlaps sideways with a similar orbital of the other carbon atom to form two weak -bonds. To form a C-H bond, the remaining sp hybrid orbital of each carbon atom overlaps with the 1s orbital of hydrogen. The orbital structure of ethyne, as shown in the figure below, illustrates the structure of the triple bond:
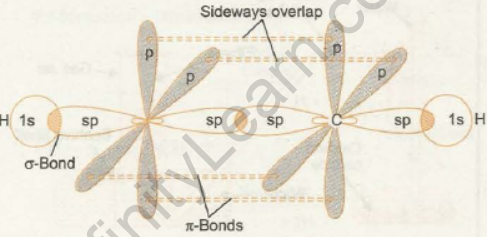
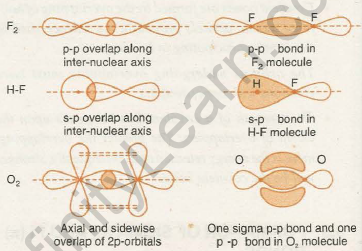
As a result, a carbon-to-carbon triple bond is composed of one sigma bond and two pi bonds. If one pi-electron cloud is depicted as lying above and below the internuclear axis representing the sigma bond, the other pi-electron cloud is depicted as lying in front and behind the line, as shown in the figure:
Also read: Important Topic of Chemistry: Alkynes-Nomenclature
FAQs
Is a triple bond possible?
In chemistry, a triple bond is a chemical bond between two atoms that involves six bonding electrons rather than the usual two in a single covalent bond. The most common triple bond found between two carbon atoms is found in alkynes. Other functional groups with a triple bond include cyanides and isocyanides.
Why are triple bonds the most powerful?
Triple bonds are stronger than double bonds because they contain two pi bonds rather than one. Each carbon atom has two hybrid sp-orbitals, one of which overlaps to form a sp-sp sigma bond with the corresponding one from the other carbon atom.
Is it true that alkanes have double bonds?
Alkanes, also known as paraffins, are a type of hydrocarbon that is completely saturated with hydrogen. They have the most covalent bonds from carbon to hydrogen because their carbon skeletons lack double and triple bonds.



