Table of Contents
A chemical change, often known as a chemical reaction, converts one or more pure compounds into one or more distinct pure ones. Chemical changes result in the synthesis of molecules that aid in the growth of our food, increase the productivity of our life, relieve heartburn, and much more. Whenever one or more compounds, known as reactants, are converted into one or more distinct substances, known as products, a chemical reaction occurs. Substances include chemical elements and compounds. In a chemical reaction, the constituent atoms of the reactants are rearranged, resulting in the production of diverse substances as products. Chemical changes are an important part of technology, society, and even life itself. Chemical processes have been studied and practised for thousands of years, including burning fuels, smelting iron, producing glass and pottery, brewing beer, and making wine and cheese. Chemical reactions can be found in the Earth’s geology, atmosphere, and oceans, as well as a variety of complicated living activities.
The idea of a chemical reaction has been around for roughly 250 years. Its roots may be traced back to early studies that categorised chemicals as elements and compounds, as well as hypotheses that explained how these processes worked. The development of the concept of a chemical reaction was crucial in defining chemistry as we know it today.
Overview
Reactants are molecules that combine to make new compounds, whereas products are newly formed compounds. We can say that chemical reactions play a significant role in a wide range of industries, cultures, and even our everyday lives. Rusting of iron, pottery, and wine fermentation, to name a few, are all examples of them in our environment. Physical changes such as precipitation, heat production, colour change, and so on all require a chemical change to occur in a chemical reaction.
Except for the nonmetals in Group VIIIA, hydrogen combines with every element in the periodic table (He, Ne, Ar, Kr, Xe, and Rn). Although it is commonly stated that carbon is included in more compounds than any other element, this is not always the case. Hydrogen is found in nearly all carbon molecules, and it forms compounds with essentially all other elements. Despite the fact that the term hydride literally refers to compounds containing an H– ion, hydrogen compounds are usually referred to as hydrides.
The reaction of oxygen with another element usually leads to the development of an oxide, which is a binary molecule. Oxidation is the name for the reaction. The oxidation reaction between oxygen and sodium, for example, creates sodium oxide. In many circumstances, an element can produce several oxides.
Trends in Chemical Reactivity with Hydrogen
Hydrogen atoms are extremely reactive. It forms hydrides with most elements (for example, sodium hydride, NaH) and decreases metallic oxides, resulting in the elemental state of the metal. The energy released by this reaction heats the surfaces of metals that do not combine with hydrogen to produce stable hydrides (e.g., platinum), which catalyse the recombination of hydrogen atoms to form hydrogen molecules.
At room temperature, molecular hydrogen can react with a wide range of elements and compounds, but the reaction rates are usually so low that they are inconsequential. This seeming inertness is due in part to the molecule’s extremely high dissociation energy. The reaction rates, on the other hand, are quite fast at high temperatures.
Reaction of Alkali Metals with Hydrogen
The chemical elements in Group 1 of the periodic table are known as alkali metals. Lithium, sodium, potassium, rubidium, cesium, and francium are alkali metals. Hydrogen is not strictly an alkali metal because it rarely shows similar characteristics, despite being included in Group 1 due to its electrical arrangement. Since these elements react with water to generate hydroxide ions, causing alkaline solutions, the word “alkali” comes from the Arabic word “al qali,” which means “from ashes.” Alkali metals have a number of characteristics in addition to being highly reactive.
- At normal temperature, all alkali metals are solids.
- The density of alkali metals is low, and some of them float in water.
- Alkali metals are soft metals. Some, like the salt, are even soft enough to cut with a knife.
Metallic hydride is generated when an alkali metal reacts with hydrogen.
Consider M as an alkali metal.
2M+H2→2MH
MH can be denoted as M++H-
Here, the transformation of hydrogen can be represented as H2+2e–→2H–
As a result, a hydrogen molecule takes two electrons to generate two hydride ions. It is known that the gain of electrons is reduced. Thus, hydrogen is reduced to hydride ions.
Note that alkali metal is oxidized to M+ions.
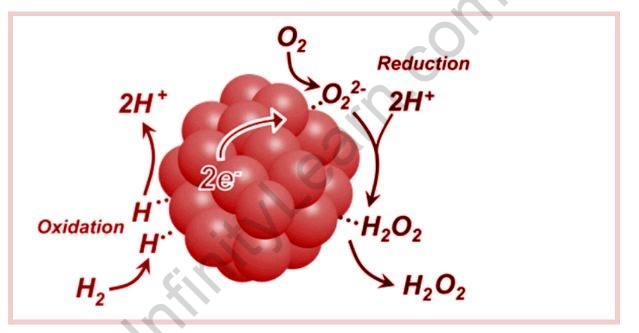
Hydrogen Peroxide and Oxygen Reaction
Energy is released when molecular hydrogen and oxygen are mixed and allowed to react, and the hydrogen and oxygen molecules can combine to form either water or hydrogen peroxide. H2O2 is a colourless liquid that dissolves in water and is commonly used as a disinfectant and bleaching agent. It is volatile or unstable and decomposes slowly, releasing water and oxygen gas. Hydrogen peroxide solutions at high concentrations are potent oxidizers and can be used as rocket fuel. Because hydrogen peroxide decomposes far more quickly in the presence of light, an opaque bottle can help slow down the process. Two hydrogen peroxide molecules decompose into two molecules of water and one molecule of oxygen gas, as well as heat energy. In most circumstances, this process is sluggish, but once a bottle of hydrogen peroxide is opened, the built-up oxygen gas is released, causing it to break down more quickly.
Also read: Sodium Hydrogen Carbonate
FAQs
Why is Hydrogen Peroxide stored in plastic containers?
When hydrogen peroxide is exposed to sunshine, it decomposes, and residues of alkali metals catalyse the process. So, it is maintained in dark, wax-lined glass or plastic containers.
Do group 1 metals react with hydrogen?
Coldwater reacts vigorously with all alkali metals. Each process releases hydrogen gas, resulting in the formation of metal hydroxide. The reaction's quickness and intensity increase as you move through the group.
Which element in Group 2 does not react with hydrogen?
The only alkaline earth metal that does not react with water is beryllium (Be). Its tiny size and high ionisation energy compared to the other elements in the group account for this.



