Table of Contents
Chemical bonding is explained using the valence bond and molecular orbital theories. A chemical bond can be formed when the orbitals of two atoms with unpaired electrons overlap. When two atomic orbitals intersect between the nuclei of two atoms, a sigma bond () is formed. When two atomic orbitals intersect outside of the space between the nuclei, pi bonds form (outside of the internuclear axis). The strongest bonds are formed when the orbitals overlap the most. Valence bond (VB) theory, together with molecular orbital (MO) theory, is one of two basic theories in chemistry that uses quantum mechanics to describe chemical bonding. A covalent bond is formed by the physical clash of half-filled valence orbitals in two or more atoms, as per VB theory.
A brief outline
Valence Bond Theory:
The crossover of half-filled atomic orbitals (each holding a single electron) yields a pair of electrons amongst both linked atoms, according to the Valence Bond Theory. When a part of one orbital and a section of a second orbital occupies the same region of space on two separate atoms, we say they overlap. A covalent bond is formed when two criteria are met: (1) an orbital on one atom intersects an orbital on another atom, and (2) the solitary electrons in each orbital unite to create an electron pair, according to valence bond theory.
The mutual interest here between the negatively charged electron pair as well as the positively charged nuclei of the two atoms creates a covalent bond that physically connects the two atoms. The level of overlap of the orbitals engaged determines the strength of a covalent bond. Orbitals with a lot of overlap form stronger bonds than orbitals with less overlap.
Important concepts
Valence Bond Theory Postulates
The valence bond theory’s main postulates are mentioned below.
- When two valence spins (half-filled) from two separate atoms overlap on one other, covalent bonds are created. As a result of this overlapping, the electron density in the intersection of two bonding atoms increases, boosting the stability of the resulting molecule.
- An atom’s valence shell has several unpaired electrons, allowing it to make many bonds with other atoms. According to the valence bond theory, the paired electrons in the valence shell need not participate in the creation of chemical bonds.
- Chemical bonds that are covalent are directed and parallel to the region corresponding to the overlapping atomic orbitals.
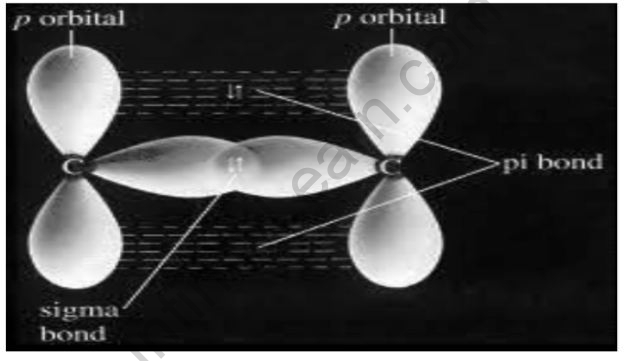
- Sigma bonds and pi bonds are distinguished by the pattern in which the atomic orbitals overlap, i.e., pi bonds are produced by overlapping along the axis having the nuclei of the two atoms, whereas sigma bonds are formed by overlapping along the axis comprising the nuclei of the two atoms.
The head-to-head collision of the atomic orbitals involved in the bond forms sigma bonds. Pi bonds, but at the other end, involve the overlapping of atomic orbitals in a parallel manner.
Applications of Valence Bond Theory
- The establishment of covalent bonds in many molecules can be addressed by the greatest overlap condition stated by the valence bond theory.
- One of the essential applications is this. The variation in the length and strength of chemical bonds in H2 and F2 molecules, for example, can be explained by differences in their overlapping orbitals.
- In an HF molecule, the covalent bond is created by the overlap of the hydrogen atoms 1s orbital and the fluorine atom’s 2p orbital.
The modern approach of valence bond theory notes
Modern valence bond theory now works in tandem with molecular orbital theory, which rejects the valence bond assumption that electron pairs are isolated between two specific atoms in a molecule and instead believes that they are distributed in sets of molecular orbitals that can span the entire molecule. The molecular orbital theory provides a simple way to predict magnetic and ionization properties, whereas valence bond theory provides similar conclusions but is more difficult. Aromatic characteristics of molecules are attributed to spin coupling of orbitals in modern valence bond theory.
The valence bond theory depicts the reorganization of electrical charge that occurs when bonds are broken and established during the course of a chemical process considerably more accurately. Valence bond theory, in an instance, correctly predicts the breakdown of homonuclear diatomic molecules into individual atoms, whereas basic molecular orbital theory predicts a mixture of atoms and ions. For example, because dihydrogen’s molecular orbital function contains an equal blend of covalent and ionic valence bond structures, it mistakenly predicts that the molecule will disintegrate into an equal mixture of hydrogen atoms and hydrogen positively and negatively ions.
Valence bond theory example (Water)
Consider a more complicated molecule, such as water. The valence-shell electron structure of each hydrogen atom is 1s1. The electron configuration of oxygen’s valence shell is 2s2, 2px2, 2py1, 2pz1. So, as long as the electrons in the oxygen and hydrogen possess opposite spins, we have two unoccupied electron locations in the oxygen that can possibly form bonds with two hydrogen atoms.
It demonstrates how the spins of the hydrogen 1s atomic orbitals must be opposing in order for them to overlap with oxygen 2p atomic orbitals and form bonds. The optimal distance/overlap among the atoms here for a minimum in PE for an O-H bond is 96 pm, which is different from the optimal distance/overlap between the atoms for an H-H bond.
Significance of valence bond theory in NEET exam
Infinity Learn has created a list of all different activities that can be found at the end of each chapter in Chemistry, as well as their solutions. Following these solutions will assist you in gaining a better grasp of the subjects covered in the NEET exam. After studying a chapter, students frequently make the error of not answering questions. Students should be able to obtain the answers to all of their questions by visiting Infinity Learn’s website and looking through these solutions.
Also read: Important Topic of Chemistry: Covalent Bond
Frequently Asked Questions
What really is the valence bond theory, and how does it work?
Chemical bonding is described by this hypothesis. According to VBT, the creation of a chemical bond between two atoms is caused by the overlap of imprecisely filled atomic orbitals. The lone electrons are shared, resulting in the generation of a hybrid orbital.
What are the drawbacks of VBT?
The valence bond theory fails to explain carbon's tetravalency and provides no insight into the energies associated with electrons. The theory implies that electrons are concentrated in specific regions.
Q: What are the advantages and disadvantages of the valence bond theory?
Ans: The VBT’s maximum overlap condition can be used to elucidate how covalent bonds are generated in a variety of compounds. The theory might provide information.
Disadvantage
Valence Bond’s idea is not without flaws. It comes with its own set of constraints. They are as follows:
- It is unable to explain carbon’s tetravalency.
- The energy of electrons is not discussed in this theory.
- The electrons are thought to be localized to specific regions, according to the assumptions.







