Calculate the Percentage of Fe2+ ions in a Sample of Ferrous Sulphate. Prepare a Solution of the given Sample having Strength Exactly Equal to 14.0 g/litre. Provided M/100 KMnO4
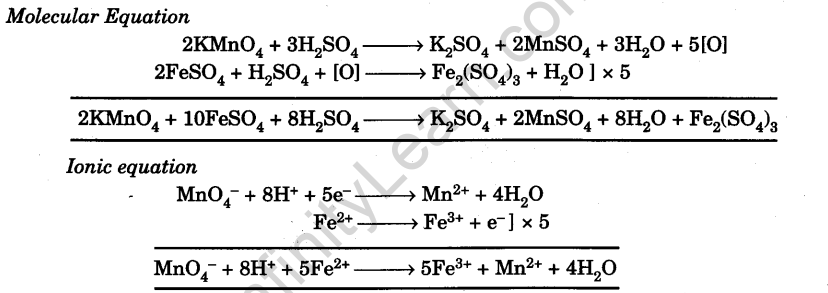
Chemical Equations
Theory
Since the given sample contains partially oxidized ferrous sulphate, it contains both ferrous ions, Fe2+ (unoxidised) and ferric ions Fe3+ (oxidised). The strength of partially oxidised sample is known. The solution of partially oxidised FeSO4 of known strength is titrated against standard KMnO4 solution to determine the molarity and strength of the unoxidised ferrous sulphate. From this the percentage oxidation of the sample can be calculated.
Indicator
KMnO4 is a self-indicator.
End Point
Colourless to permanent pink (KMnO4 in burette).
Procedure
- Weigh exactly 3.50 g of the given sample of ferrous sulphate on a watch glass and dissolve in water to prepare exactly 250 ml of solution using a 250 ml measuring flask. Rinse and fill the pipette with prepared ferrous sulphate solution and pipette out 20.0 ml of it in a washed titration flask.
- Rinse and fill the burette with the M/100 KMnO4 solution.
- Add one test-tube (~ 20 ml) full of dilute sulphuric acid (- 2 M) to the solution in titration flask.
- Note the initial reading of the burette.
- Now add KMnO4 solution from the burette till a permanent light pink colour is imparted to the solution in the titration flask on addition of a last single drop of KMnO4 solution.
- Note the final reading of the burette.
- Repeat the above steps 4—5 times to get three concordant reading.
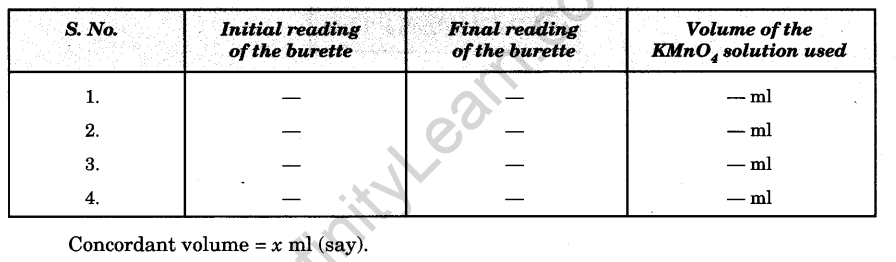
Observations
Weight of watch glass =……. g
Weight of watch glass + Mohr’s salt =…………..g
Weight of mixture = 3.50 g
Volume of solution prepared = 250 ml
Molarity of KMnO4 solution =M/100
Volume of oxalate solution taken for each titration = 20.0 ml.
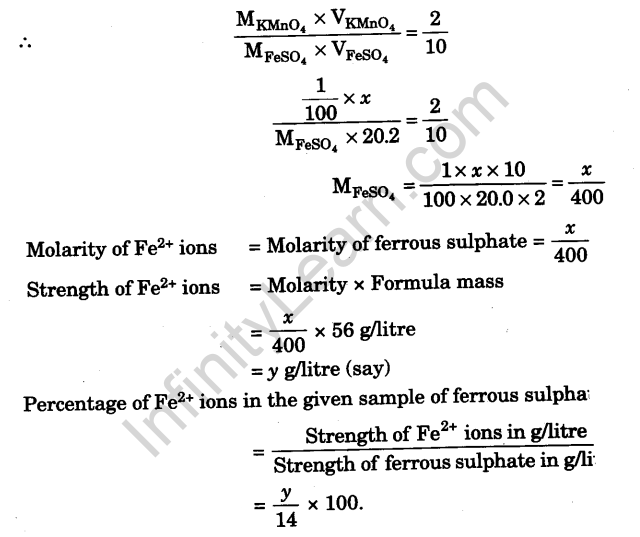
Calculations
Volume of M/100 KMnO4 solution required for the oxidation of 20.0 ml of the prepared ferrous sulphate solution = x ml.
From the equations it is clear that 2 moles of KMnO4 react with 10 moles of ferrous sulphate.

Exercises
- Prepare a standard solution of M/50 FeSO4(NH4)2SO4.6H20 (Mohr’s salt). Using this solution find out the molarity of the given solution of KMn04.
- Prepare M/50 solution of oxalic acid. Using this solution find out the molarity and strength of the given solution of KMnO4.
- Prepare a solution of ferrous ammonium sulphate containing exactly 4.9 g of the salt per 250 ml of solution. Using this solution determine the concentration of KMnO4 in g/litre in the given solution.
- Prepare M/20 solution of oxalic acid. Using this solution find out percentage purity of impure sample of KMnO4, 3.5 g of which have been dissolved per litre.
- Prepare M/50 ferrous ammonium sulphate solution. With its help, find out the percentage purity of impure sample of KMnO4, 3.6 g of which have been dissolved per litre.
- Prepare M/20 oxalic acid solution. You are provided two solutions of KMnO4, A and B. Find out volumetrically which solution, (A or B) is more concentrated. Report the strength of more concentrated solution in g/litre.
- You are provided with a solution of alkali metal permanganate, AMn04 containing 3.15 g of it per litre of the solution. Prepare M/20 oxalic acid solution and using this solution determine the atomic mass of the alkali metal ‘A’.
- Determine volumetrically the percentage purity of a given sample of sodium oxalate. Provided M/50 KMnO4 solution.

