Table of Contents
- Atomic Size
- Summary
- What’s Next?
In the previous segment of the chapter ‘Periodic Classification of Elements’, we learnt about
valency. In this segment, let us get introduced to atomic size.
What is Atomic size?
- Atomic size indicates the size of a single atom in that element.
- It is measured in terms of its radius by measuring the distance from the centre of the nucleus to the last shell of the atom.
For example, in the hydrogen atom, the distance from the centre of its nucleus till this last electron shell gives us its atomic size.
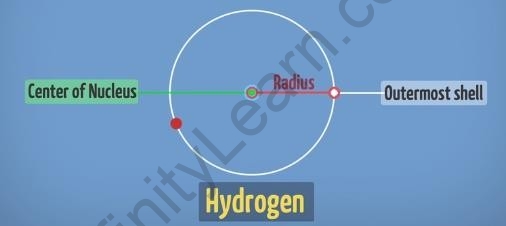
Atomic size of the hydrogen atom
- The number of shells in the atoms increases from top to bottom in a group. The number of shells in each atom is different.
- The distance from the nucleus to the last shell increases from top to bottom in a group. Hence, the atomic radii increase from top to bottom in a group.
- The atomic size of all the elements in the period will always be the same because the number of shells is the same across the period.
- The number of shells is the same but the number of valence electrons is different. Due to more electrons in the same number of shells, the force of attraction from the nucleus will increase and the atom shrinks in size.
- This gives rise to a decrease in the radii of atoms. So, the atomic size decreases from left to right in the period.

