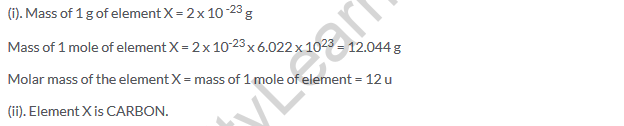Table of Contents
Lakhmir Singh Chemistry Class 9 Solutions Chapter 3 Atoms and Molecules
Text Book Solutions – Lakhmir Singh and Manjit Kaur – Chemistry
Lakhmir Singh Class 9 CBSE Chemistry Text Book PAGE No 128
Solution 01
International Union of Pure and Applied Chemistry
Solution 02
(a) Law of conservation of mass – Antonie Lavoisier
(b) Law of constant proportions – Joseph Proust
Solution 03
(a) Law of conservation of mass.
(b) Law of constant proportions.
Lakhmir Singh Class 9 CBSE Chemistry Text Book PAGE No 129
Solution 04
John Dalton
Solution 05
Atoms can neither be created nor destroyed.
Solution 06
The elements consist of atoms having fixed mass, and that the number and kind of atoms of each element in a given compound is fixed.
Solution 07
Kanad ; ‘parmanu’.
Solution 08
Law of Conservation of mass and law of constant proportions.
Solution 09
Law of constant proportions.
Solution 10
Law of Conservation of mass.
Solution 11
Atoms are the building blocks of matter.
Solution 12
The size of an atom indicated by its radius which is called ‘atomic radius’.
Solution 13
The radius of an atom is usually expressed in ‘nanometers’.
Solution 14
1 nanometer = 10-9 m
Solution 15
‘nm’ represents nanometer.
Solution 16
Because they are very very small.
Solution 17
False ; it is Co
Solution 18
The molecular mass of a substance is the relative mass of its molecule as compared with the mass of a Carbon-12 atom taken as 12 units.
Solution 19
This means that a molecule of oxygen is 32 times heavier than 1/12 of a Carbon-12 atom.
Solution 20
(a) 1:8
(b) Conservation of mass
Solution 21
(a) Carbon is used as a standard for atomic mass scale.
(b) Atom with 6 neutrons and 6 protons in its nucleus so that its mass number is 12.
(c) Mass = 12 u
Solution 22
The major drawback of Dalton’s atomic theory is that atoms were thought to be indivisible. But, it is not true since atoms are divisible.
Solution 23
No, the statement is not valid because atoms can be divided into subatomic particles called electrons, protons and neutrons.
Solution 24
Yes, ‘THE SCANNING TUNNELLING MICROSCOPE’ enables people to see atoms. This microscope can produce computer generated images of the surface of elements which show the individual atoms. The atoms show up as blurred images.
Solution 25
The symbol of element is the “first letter” or “first letter and another letter” of the English name or Latin name of the element.
For example, symbol of Hydrogen is “H” and symbol of Calcium is “Ca”.
Solution 26
(a)Ca, Mg
(b)Cu, Hg
Solution 27
Hydrogen- H, Helium-He, Lithium-Li , Beryllium-Be, Boron-B
Solution 28
Sodium – Na
Potassium – K
Iron – Fe
Copper – Cu
Mercury – Hg
Silver – Ag
Solution 29
Hg – Mercury
Pb – Lead
Au – Gold
Ag – Silver
Sn – Tin
Solution 30
The number of atoms present in one molecule of an element is called atomicity of that element.
For example, atomicity of sodium is 1 and that of nitrogen is 2.
Solution 31
(a) Oxygen = 2
(b) Ozone = 3
(c) Neon = 1
(d) Sulphur = 8
(e) Phosphorus = 4
(f) Sodium = 1
Solution 32
A chemical formula represents the composition of a molecule of the substance in terms of the symbols of the elements present in the molecule. It is also known as molecular formula.
Chemical formula of element – H2 for hydrogen
Chemical formula of compound – H2O for water
Solution 33
(a). Water- H2O; Elements present are Hydrogen and Oxygen.
(b).Ammonia- NH3; Elements present are Nitrogen and Hydrogen.
(c).Methane-CH4; Elements present are Carbon and Hydrogen.
(d).Sulphur dioxide-SO2; Elements present are Sulphur and Oxygen.
(e).Ethanol-C2H5OH; Elements present are carbon, hydrogen and oxygen.
Solution 34
2N represents two separate atoms of nitrogen and N2 represents one molecule of nitrogen.
Lakhmir Singh Class 9 CBSE Chemistry Text Book PAGE No: 130
Solution 35
(a) O- one atom of oxygen
(b) 2O- two separate atoms of oxygen
(c) O2-one molecule of oxygen
(d)3O2-three molecules of oxygen
Solution 36
H2 represents two atoms of hydrogen, one atom of sulphur and four atoms of oxygen.
Solution 37
(a) Oxygen gas occurs as a diatomic molecule in nature.
(b) Noble gases occur as monoatomic gases in nature.
Solution 38
2H represents two separate atoms of hydrogen and H2 represents one molecule of hydrogen.
Solution 39
(a) N – one atom of nitrogen
(b) 2N – two separate atoms of nitrogen
(c) N2 – one molecule of nitrogen
(d) 2N2 – two molecules of nitrogen
Solution 40
Significance of formula of a substance-
1. Formula represents the name of the substance.
2. Formula represents one molecule of a substance.
3. Formula gives the number of atoms of each element present in one molecule.
4. Formula also represents one mole of molecules of the substance.
Solution 41
Significance of the formula H2O-
1. H2O represents water.
2. It represents one molecule of water.
3. H2O also represents 6.022 x 1023 molecules of water.
4. It represents 18gm of water.
Solution 42
Molecular formula of glucose = C6H12O6
Molecular mass of glucose = (6xC)+(12xH)+(6xO) =72 + 12 + 96 = 180u.
Solution 43
(a).Molecular mass of Hydrogen (H2) = 2 x H = 2 x 1 u = 2 u
(b).Molecular mass of oxygen (O2) = 2 x O = 2 x 16 u = 32 u
(c).Molecular mass of chlorine (Cl2) = 2 x Cl = 2 x 35.5 = 71 u
(d).Molecular mass of Ammonia (NH3) = 1 x N + 3 x H = 14 + 3 = 17 u
(e).Molecular mass of carbon dioxide (CO2) = 1 x C + 2 x O = 12 + 32 = 44 u
Solution 44
(a). Molecular mass of methane (CH4) = 12 + 4 = 16 u
(b). Molecular mass of ethane (C2H6) = 2 x 12 + 6 x 1 = 30 u
(c). Molecular mass of ethane (C2H4) = 2 x 12 + 4 x 1 = 28 u
(d). Molecular mass of ethyne (C2H2) = 2 x 12 + 2 x 1 = 26 u
Solution 45
(a) Molecular mass of Methanol(CH3OH) =
1xC + 3xH + 1xO +1xH = (12+3+16+1)u = 32u
(b) Molecular mass of Ethanol (C2H5OH) = 2xC + 5xH + 1xO +1xH
= (24 + 5 + 16 + 1) = 46u
Solution 46
Molecular mass of ethanoic acid (CH3COOH)
= 1xC + 3xH + 1xC + 2xO +1xH = 12+3+12+32+1 = 60u
Solution 47
Molecular mass of Nitric acid (HNO3) = 1xH + 1xN + 3xO
= (1 + 14 +48) u = 63 u
Solution 48
Molecular mass of chloroform (CHCl3) = 1 x C + 1 x H + 3 x Cl
= (12 + 1 + 106.5)u = 119.5 u
Solution 49
Molecular mass of hydrogen bromide (HBr) = 1 x H + 1 x Br
= (1 + 80) u = 81u
Solution 50
(a).Molecular mass of hydrogen sulphide (H2S) = 2xH + 1xS
= (2+32) u = 34u
(b). Molecular mass of Carbon disulphide (CS2) = 1xC + 2xS = (12+2 x 32) u = 76 u
Solution 51
Law of conservation of mass by LAVOISIER states that: “Mass can neither be created nor be destroyed in a chemical reaction”. So, in a chemical reaction, the total mass of reactants must be equal to the total mass of products.
For example: When calcium carbonate is heated, a chemical reaction takes place to form calcium oxide and calcium carbonate. If 100 gms of calcium carbonate is decomposed completely, then 56 gms of calcium oxide and 44 gms of carbon dioxide are formed.
In the above example: the total mass of products = 56 gms (CaO) + 44gms (CO2) = 100 gms.
As total mass of products is equal to the total mass of reactant so, the law of conservation of mass is satisfied.
Solution 52
Law of constant proportion given by PROUST states that “A chemical compound always consists of the same elements combined together in the same proportion by mass.”
For example: If we decompose 100 gms of pure water by passing electricity through it, then 11 gms of hydrogen and 89 gms of oxygen are obtained. Now, if we repeat this experiment by taking pure water from different sources (like river, sea, well, etc.), the same masses of hydrogen and oxygen elements are obtained in each case. They are always combined together in the same constant proportion of 11:89 or 1:8 by mass. And this is the law of constant proportions.
Solution 53
[a]. Postulates of Dalton’s atomic theory:
a) All the matter is made up of very small particles called ‘atoms’.
b) Atoms cannot be divided.
c) Atoms can neither be created nor be destroyed.
d) Atoms are of various kinds. There are as many kinds of atoms as are elements.
e) All the atoms of a given element are identical in every respect, having the same mass, size and chemical properties.
f) Atoms of different elements differ in mass, size and chemical properties.
g) The ‘number’ and ‘kind’ of atoms in a given compound is fixed.
h) During chemical combination, atoms of different elements combine in small whole numbers to form compounds.
i) Atoms of the same elements can combine in more than one ratio to form more than one compound.
[b]. The postulate “The elements consists of atoms and that atoms can neither be created nor destroyed” can be used to explain the law of conservation of mass.
[c]. The postulate “The elements consist of atoms having fixed mass, and that the number and kind of atoms of each element in a given compound is fixed” can be used to explain the law of constant proportions.
Solution 54
(a).Significance of symbol of element –
i. It represents name of the element.
ii. It represents one atom of the element.
iii. It represents a definite mass of the element.
iv. It represents one mole of atoms of the element.
For example – C represents one atom of the element Carbon. It also represents 12 gms of Carbon.
(b). Significance of symbol H –
i. It represents Hydrogen element.
ii. It represents one atom of Hydrogen element.
iii. It represents one mole of Hydrogen atoms.
iv. It represents 2 gms of Hydrogen.
Solution 55
a) An atom is the smallest particle of an element that can take part in a chemical reaction. They usually exist in combination with the atoms of same element or another element.
b) A molecule is an electrically neutral group of two or more atoms chemically bonded together.
For example- Ozone gas has three oxygen atoms combined together, so ozone exists in the form of O3 molecule.
c) The molecule of an element contains two or more similar atoms chemically bonded together.
For example- A molecule of hydrogen element consists of 2 hydrogen atoms combined together.
Whereas the molecule of compound contains two or more different type of atoms chemically bonded together.
For example- The molecule of hydrogen chloride(HCl) contains two different type of atoms, i.e. H and Cl.
Solution 56
(a) One atomic mass unit is defined as exactly one-twelfth the mass of an atom of carbon-12. Its symbol is ‘u’.
(b) The atomic mass of an element is the relative mass of its atom as compared with the mass of a carbon-12 atom taken as 12 units.
(c) It means that one atom of oxygen is 16 times heavier than 1/12 of a C-12 atom.
Lakhmir Singh Class 9 CBSE Chemistry Text Book PAGE NO 131
Solution 75
According to question-
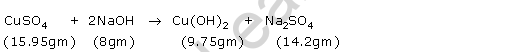
Clearly, in this case
total mass of reactants = (15.95 gm + 8 gm) = 23.95 gm
total mass of products = (9.75 gm + 14.2 gm) = 23.95 gm
Hence, Law of conservation of mass is valid here.
Solution 76
According to question-
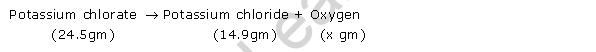
Let, x gm of oxygen is formed
Then, according to law of conservation of mass-
24.5 gm = 14.9 gm + x gm
So, x = (24.5 � 14.9) gm = 9.6 gm.
Thus, 9.6 gm of oxygen is formed in the reaction
Solution 77
According to question-
Reaction 1-
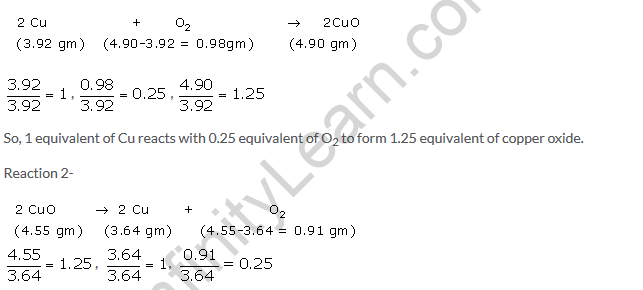
Here again, one can see that 1.25 equivalent of CuO decomposed to form 1 equivalent of Cu and 0.25 equivalent of oxygen.
Hence, law of constant proportion is verified.
Solution 78
According to question-
![]()
i.e. three equivalents of Mg reacts with 2 equivalents of O2 to form 1 equivalent of MgO.
When mass of Mg = 3x = 24 gm
So, x = 8 gm
Then, mass of oxygen required = 2x = 16 gm
Lakhmir Singh Class 9 CBSE Chemistry Text Book PAGE NO: 132
Solution 79
When 5 gm of calcium is burnt in 2 gm of oxygen, then 7 gm of calcium oxide is formed. So, calcium and oxygen combine in the fixed proportion of 5:2 by mass.
Now, when 5 gm of calcium is burnt in 20 gm of oxygen, then also 7gm of calcium oxide will be formed because chemical reactions follows law of constant proportion.
As a result, 18 gm of oxygen will be left unreacted.
Solution 80
According to question-
![]()
(a) Liquid X – Water
Gas Y – Oxygen
Gas Z – Hydrogen
(b) mass of Z / mass of Y = 22gm / 178 gm = 1:8
(c) Law of constant proportion is illustrated by this example.
(d) Two sources of liquid X – Sea, Well
(e) Gas Y (oxygen) is necessary for breathing.
Solution 81
According to question-
![]()
(a) Solid P – Calcium Carbonate (CaCO3)
Gas Q – Carbon dioxide (CO2)
Solid R – Calcium oxide (CaO)
(b) Total mass of Q and R = 22gm + 28gm = 50gm
(c) Total mass of Q and R (50gm) is equal to mass of reactant (50gm).
(d) The law of conservation of mass is followed, i.e. total mass of product is equal to mass of reactant.
(e) Law of conservation of mass is illustrated by the example.
Lakhmir Singh Class 9 CBSE Chemistry Text Book PAGE No 150
Solution 01
Ions
Solution 02
(a). Anions ; (b). Cations
Solution 03
The formula mass of an ionic compound is the relative mass of its ‘formula unit’ as compared with the mass of a Carbon-12 atom taken as 12 units.
Lakhmir Singh Class 9 CBSE Chemistry Text Book PAGE NO 151
Solution 04
(a) Anions are formed by the gain of electrons by atoms
(b) Cations are formed by the loss of electrons by atoms
Solution 05
(a) False
(b) True
Solution 06
(a). Calcium oxide – CaO
(b). Magnesium hydroxide – Mg(OH)2
Solution 07
Valency of element Z = 3
Valency of oxygen = 2
So, formula of oxide of element = Z2O3
Solution 08
Its Na+ , the sodium ion.
Solution 09
Its Cl-, the chloride ion.
Solution 10
(a) Anion
(b) Cation
(c) Ion
(d) Electrons ; protons
(e) Protons ; electrons
Solution 11
Water is made up of Hydrogen and oxygen.
Valency of hydrogen is +1 ; Valency of oxygen is -2.
Chemical formula of water is H2O.
Solution 12
![]()
So, chemical formula of ammonia is NH3.
Solution 13
![]()
Chemical formula of sulphur dioxide is SO2.
Solution 14
According to question-
![]()
Name and formula of the resulting compound is Carbon disulphide; CS2.
Solution 15
As the valency of element X is 4 and that of Y is 1, so the resulting formula is XY4.
Solution 16

Solution 17
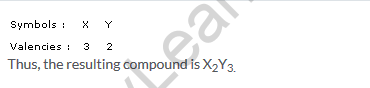
Solution 18

Solution 19
(a). Bromide of element-
As valency of bromine is -1 and that of element X is +2 so, the resulting compound is XBr2.
(b). Oxide of element-
As valency of oxygen is -2 and that of element is +2 so, the resulting compound is XO.
Solution 20
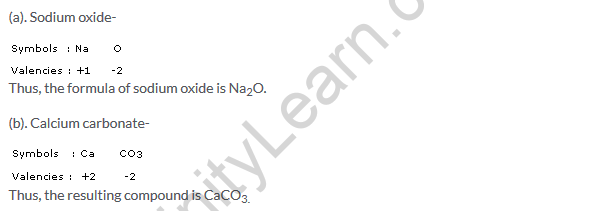
Solution 21
(a).Molecular mass of Na2O = (2 x Na) + (1 x O) = (2 x 23) + (1 x 16) = 62u
(b). Molecular Mass of Al2O3 = (2 x Al) + (3 x O) = (2 x 27) + (3 x 16) = 102u
Solution 22
(a). CuSO4 : Copper sulphate; Cu+2 and SO4-2
(b). (NH4)2SO4 : Ammonium sulphate; NH4+ and SO4-2.
(c). Na2O : Sodium oxide; Na+ and O-2
(d). Na2CO�3 : Sodium carbonate; Na+ and CO4-2.
(e). CaCl2 : Calcium chloride; Ca+2 and Cl-.
Solution 23
(a). CH3COONa : Na+ (cation) and CH3COO- (anion)
(b). NaCl : Na+ (cation) and Cl- (anion)
(c). H2 : It is a covalent molecule. So, cation and anion are not present.
(d). NH4NO3 : NH4+ (cation) and NO3- (anion)
Solution 24
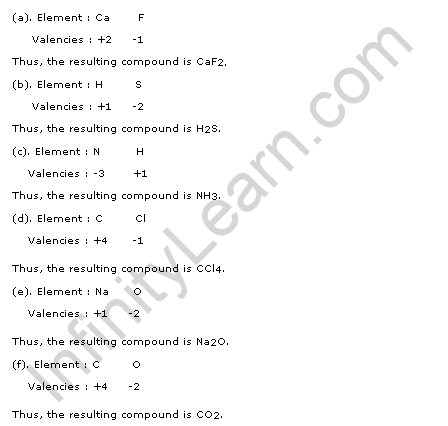
Solution 25
i. Ionic compounds- The compounds which are formed by combination of metals and non-metals are called ionic compounds. For ex: CaCl2 and CaCO3.
ii. Molecular compounds- These compounds are formed by the combination between two non-metal elements. For ex. HCl and H2S.
Lakhmir Singh Class 9 CBSE Chemistry Text Book PAGE No 152
Solution 26

Solution 27
(a)A cation is formed by the loss of one or more electrons by an atom. For ex. Magnesium loses 2 electron to form Mg+2.
An anion is formed by the gain of one or more electrons by an atom. For Ex. Chlorine loses one electron to form Cl-.
(b) (i). Na2S
(ii). Cu (NO3)2
Solution 28
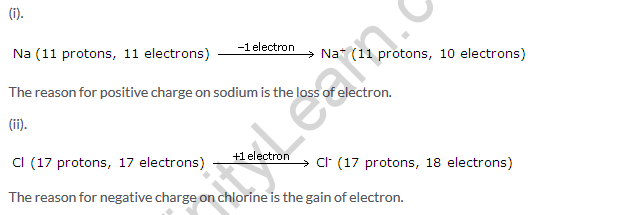
Solution 29
(a). Simple ions: Br- and Na+ ; Compound ions: NH4+ and Al+3
(b). (i). YCl4 (ii). YO2 (iii). Y (SO4)2 (iv). Y (CO3)2 (v). Y (NO3)4
Solution 30
(a). The simplest combination of ions that produces an electrically neutral unit, is called ‘formula unit’ of the ionic compound.
Formula unit of sodium chloride – NaCl
Formula unit of magnesium chloride – MgCl2
(b).
(i). Formula Mass of Calcium chloride (CaCl2) = 1xCa + 2xCl = (40+71) u = 111 u
(ii). Formula Mass of Sodium carbonate (Na2CO3) = 2xNa + 1xC + 3xO= (2×23 + 1×12 + 3×16) u = 106 u
Lakhmir Singh Class 9 CBSE Chemistry Text Book PAGE NO 153
Solution 46
(a). Let the valency of element A be y, then
2y + 5(-2) = 0
So, y = valency of element A = 5
(b). As valency of element A is 5 and valency of chlorine is -1,
So, the formula of chloride of A is ACl5.
Solution 47
Valency of X –
(i). In H2X : -2
(ii).In CX2 : -2
(iii).In XO2 : +4
(iv).In XO3 : +6
Solution 48
Let the valency of X be y, then
2 x (+3) + 3 x y = 0
So, valency of X = y = -2
As valency of Mg is +2 and that of X is -2 so the formula of Magnesium salt of X will be MgX.
Solution 49
According to formula M2CO3, valency of M is +1.
(a). formula of iodide = MI (as valency of iodine is -1)
(b). formula of nitride = M3N (as valency of nitrogen is -3)
(c). formula of phosphate = M3PO4
Solution 50
(a). Anion will be formed by element X ; Symbol : X-
(b). (i). No. of protons in X = 17
(ii). No. of electrons in X = 18
(iii). No. of neutrons in X = 18
(c). Cation will be formed by element Y ; Symbol : Y+
(d). (i). No. of protons in Y = 11
(ii). No. of electrons in Y = 10
(iii). No. of neutrons in Y = 12
(e).Atomic mass of X = No. of protons(17) + No. of neutrons(18) = 35 u
Atomic mass of Y = No. of protons (11) + No. of neutrons (12) = 23 u
(f). Element X is Chlorine (Cl).
Element Y is Sodium (Na).
Lakhmir Singh Class 9 CBSE Chemistry Text Book PAGE NO: 172
Solution 1
A mole
Solution 2
1 mole
Solution 3
![]()
Solution 4
![]()
Solution 5
![]()
Solution 6
Avogadro number
Solution 7
Given mass of oxygen = 12g
Molar mass of oxygen = 32g
No. of moles = Given mass / Molar mass = 12g /32g = 0.375
Solution 8
No. of moles = 3.6g / 18g = 0.2 mole
Solution 9
Mass of 0.2 moles of oxygen atoms = 0.2 x 16 = 3.2g
Solution 10
Mass of 2 moles of nitrogen atoms = 2 x 14 = 28g
Solution 11
Given mass of CaCO3 = 10g
Molar mass of CaCO3 = 1xCa + 1xC + 3xO = (40+12+48)gm = 100gm
So, no. of moles of CaCO3 = Given mass/Molar mass = 10/100 = 0.1 moles
Solution 12

Solution 13
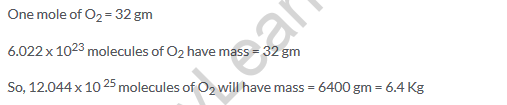
Solution 14
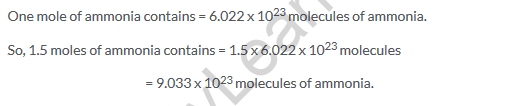
Solution 15
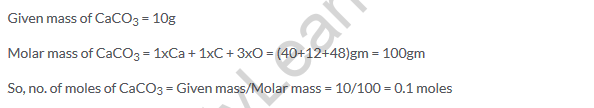
Solution 16
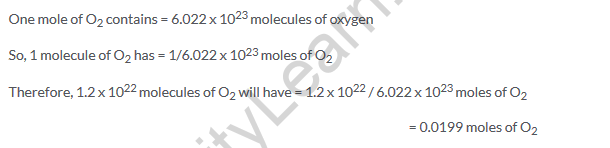
Solution 17

Solution 18
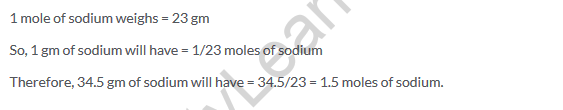
Solution 19
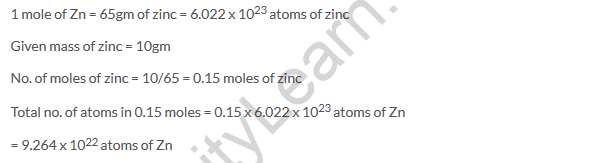
Solution 20
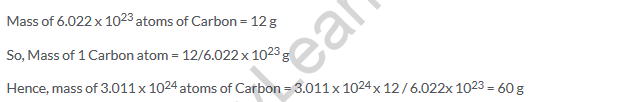
Solution 21
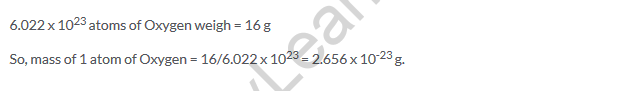
Lakhmir Singh Class 9 Chemistry Text Book Page No 173
Solution 22
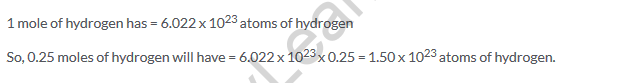
Solution 23

Solution 24
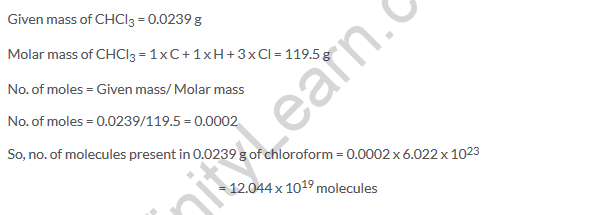
Solution 25

Solution 26
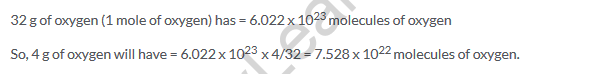
Solution 27
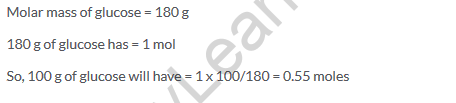
Solution 28
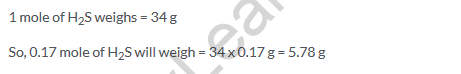
Solution 29
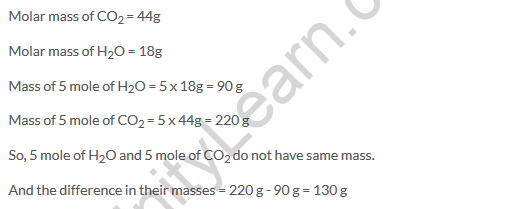
Solution 30

Solution 31

Solution 32
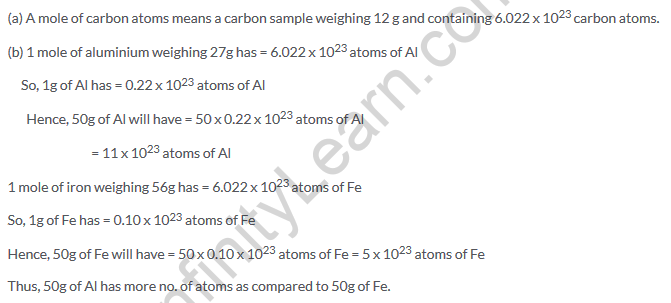
Solution 33

Solution 34

Solution 35
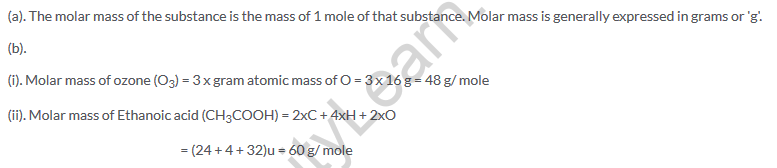
Lakhmir Singh Class 9 Chemistry Text Book Page No 174
Solution 43

Solution 44
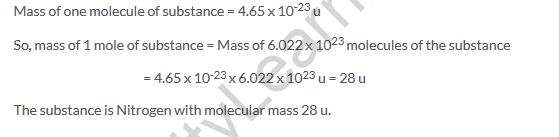
Solution 45
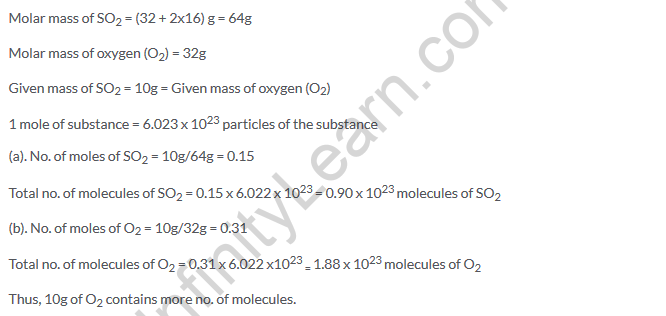
Solution 46

Solution 47

Solution 48

Solution 49

Solution 50