To Prepare a Sample of Dibenzalacetone
Theory
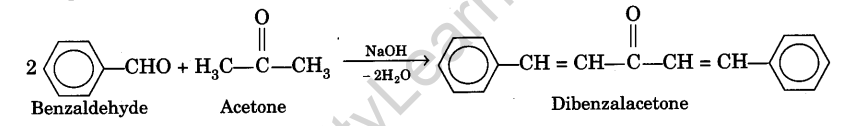
The preparation of dibenzal acetone is an example of Claisen-Schmidt reaction. This reaction takes place between aromatic aldehydes and aliphatic ketones in presence of sodium hydroxide. Two moles of benzaldehyde condense with one mole of acetone to give dibenzalacetone. The chemical equation can be written as :
Apparatus
Conical flask (100 ml), beaker (250 ml), test-tube, funnel, filter-papers, etc.
Chemicals Required
Benzaldehyde = 2.5 ml
Acetone = 1.0 ml
10% NaOH solution = 5 ml
Rectified spirit = 25 ml
Procedure
- Take a conical flask (100 ml) and add 2.5 ml benzaldehyde, 1.0 ml of acetone and 25 ml of methylated spirit. Cork the flask and shake to obtain a clear solution.
- Take 5 ml of 10% NaOH solution in a test-tube and add this to conical flask drop by drop with shaking of the flask. Maintain the temperature of the reaction mixture between 20-25°C during addition of sodium hydroxide solution.
- Cork the flask again and shake vigorously for about 10 minutes, releasing pressure from time to time.
- Allow it to stand for about 20 minutes at room temperature and then cool in ice water for a few minutes.
- Filter the yellow coloured solid and wash it with water to remove traces of alkali.
- Recrystallization of dibenzalacetone.
Dissolve the above yellow coloured crude solid in minimum amount of hot rectified spirit and then allow it to cool slowly. Pale yellow crystals of dibenzalacetone separate out. Filter the crystals and dry. - Weigh and record its yield and melting point.
Result
Weight of dibenzalacetone obtained =…………g
Melting point of dibenzalacetone is………°C
Note: (Approximate expected yield of dibenzalacetone is 1.5 g)
The melting point of dibenzalacetone is 112°C.
Precautions
- Add NaOH dropwise to the reaction mixture with constant shaking and maintaining the temperature around 20°C.
- Wash the ppt. with water to remove traces of sodium hydroxide sticking to them.
- Use minimum amount of rectified spirit to dissolve crude sample for crystallisation.

