Table of Contents
Section A
1.Suggest a quantitative method for the estimation of the gas which protects us from UV rays coming from the Sun.
2.In the two bases given below, which, one is present in RNA and which one is present in DNA?
(i) Thymine (ii) Uracil
3.Give the structure and IUPAC name of the compound di-sec. butylketone.
4.Why is an increase in temperature observed on mixing chloroform with acetone?
5.What do you mean by percentage by weight?
Section B
6.Explain, how can you determine the atomic mass of an unknown metal if you know its mass density and the dimensions and type of the unit cell of its crystal.
7.Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
8.Identify the major product of acid-catalysed dehydration of
- 1-methyl cyclohexanol.
- butan-l-ol.
9 .Calculate the packing efficiency of a metal crystal for a simple cubic lattice. (2)
10. Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine, respectively in the presence of Lewis acid catalysts. But why does preparation of aryl iodides requires presence of an oxidising agent?
What is the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark?
Or
Explain, why
- alkyl halides, though polar, are immiscible with water?
- Grignard reagent should be prepared under anhydrous conditions?
Section C
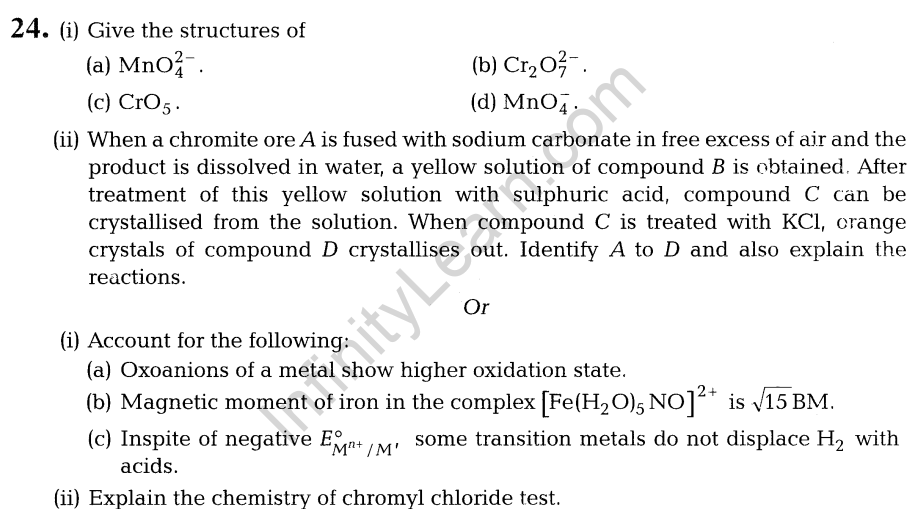
11. (i) Maximum rates attained (Vmax) for an enzyme catalysed reaction at different temperature are given below.
| T/°C | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 |
| Vmax x 103 Ms”1 | 1.0 | 1.7 | 2.3 | 2.6 | 3.2 | 4.0 | 2.6 | 0.2 |
Comment about the enzyme activity.
- Write an expression to calculate time required for completion of zero order reaction.
- In a reaction, if the concentration of reactant A is tripled, the rate of reaction becomes twenty seven times. What is the order of the reaction?
12. (i) What is tincture of iodine? What is its use?
(ii) What is the advantage of using anti-histamines over antacids in the treatment of acidity? As a chemist, would you sell anti-histamines over antacids? Why?
13. Which one of the following has the highest dipole moment? Explain.
- CH2C12
- CHC13
- CC14
14. (i) Write reactions of the final alkylated product of aniline with excess of methyl iodide in the presence of sodium carbonate solution.
(ii) Explain, why the pKb of dimethylamine is less than that of methylamine
15. Vapour pressure of chloroform (CHC13 ) and dichloromethane (CH2C12) at 298 K are 200 mm Hg and 415 mm Hg, respectively.
- Calculate the vapour pressure of the solution prepared by mixing 25.5 g of CHC13 and 40 g of CH2C12 at 298 K.
- Calculate the mole fractions of each component in vapour phase. (l)
Or
Two liquids A and B form ideal solution. At 300K, the vapour pressure of a solution containing 1 mole of A and 3 moles of B is 500 mm of Hg. At the same temperature, if one more mole of B is added to this solution, the vapour pressure of the solution increases by 10 mm of Hg. Calculate the vapour pressures of A and B in their pure states.
16.Explain the terms, primary and secondary structure of proteins. What is the difference between a-helix and p-pleated sheet of proteins?

18. (i) What type of building blocks are present in the structure of zeolites? What is this structure called?
(ii) Why does leather get hardened after tanning?
(iii) Differentiate between peptisation and coagulation.
19. (i) Which xenon compound is isostructural with ICI4
(ii) Draw the structure of phosphinic acid
(iii) Write a chemical reaction for the use of phosphinic acid as a reducing agent,
20. Write balanced chemical equations for the following reactions.
- Reaction of Cl2 with cold and dilute NaOH .
- Thermal decomposition of ammonium dichromate.
- When phosphine is passed through mercuric chloride solution.
21.Write the names and structures of the monomers of the following polymers.
(i) Orion (ii) Neoprene (iii) Dacron
22.(i) What happens to the colour of coordination compound [Ti(H20)6]Cl3, when heated gradually?
(ii) Give the electronic configuration of the d-orbitals of Ti in [Ti(H20)6]3+ ion and explain, why this complex is coloured? (Atomic number of Ti = 22)
(iii) Give evidence that [Co(NH3 )5Cl]S04 and [Co(NH3)5 S04 ]C1 are ionised isomers.
Section D
23. A washer woman, while washing a miner’s, overalls, she noticed that sand and similar dirt fell to the bottom of the washtub. What was peculiar, the copper bearing compounds that had come to the clothes from the mines, were caught in the soap-suds and so they came to the top. One of her clients was a chemist, Mrs Carrie Everson. The washer woman told her experience to Mrs Everson.
Based on the above passage, answer the following questions.
(i) From the observation of washer woman, Mrs Everson devised a method of concentration of copper ore. Name the method.
(ii) What were the disadvantages of earlier methods of concentration?
(iii) What values are exhibited by washer woman?
Section E
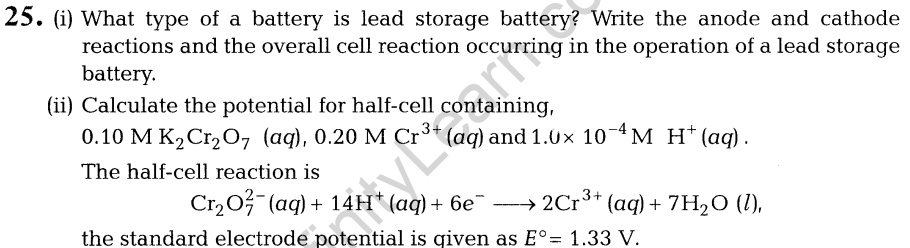
26. (i) Write chemical equations to illustrate the following name bearing reactions
- Stephen reaction
- Wolff-Kishner reduction
(ii) Give chemical tests to distinguish between the following pairs of the compounds.
- Propanol and propanone
- Acetophenone and benzophenone
- Ethanol and benzoic acid
Or
(i) How will you bring about the following conversions?
- Ethanol to 3-hydroxybutanal (1)
- Benzaldehyde to benzophenone (l)
(ii) An organic compound A has the molecular formula C8H1602. It get hydrolysed with dilute sulphuric acid and gives a carboxylic acid B and an alcohol C. Oxidation of C with chromic acid also produced B. C on dehydration reaction gives but-l-ene. Write equations for the reactions involved.



