You are Provided with a Partially Oxidised Sample of Ferrous Sulphate (FeSO4.7H20) Crystals. Prepare a Solution by Dissolving 14.0 g of these Crystals per litre and Determine the Percentage Oxidation of the given Sample. Given M/100 KMnO4 Solution
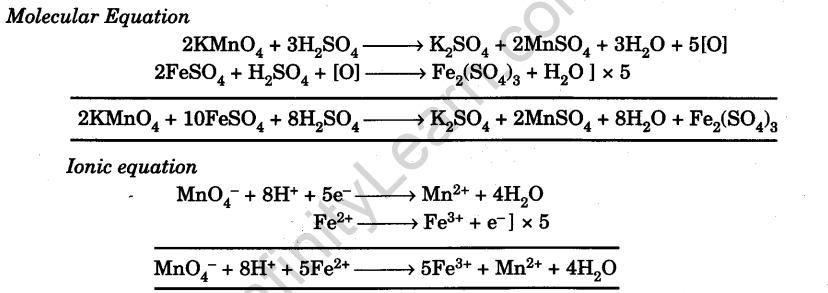
Chemical Equations
Theory
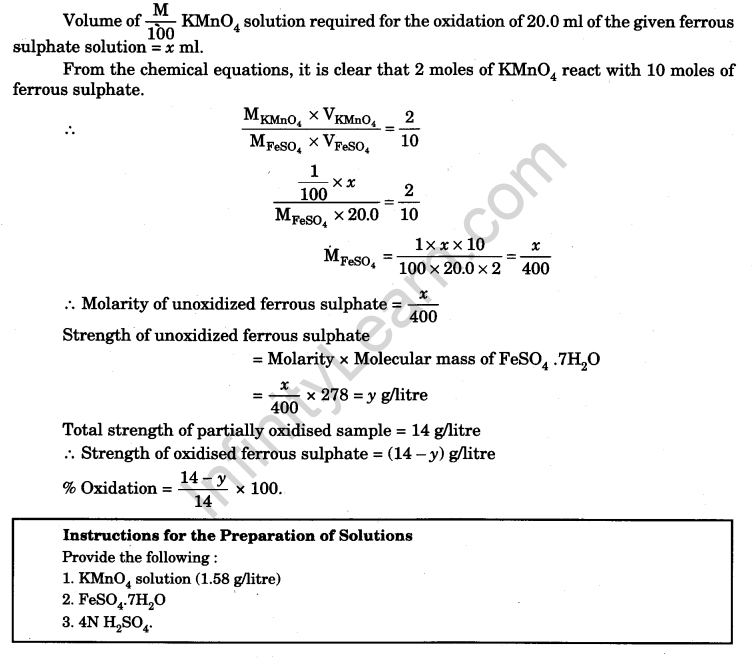
Since the given sample contains partially oxidized ferrous sulphate, it contains both ferrous ions, Fe2+ (unoxidised) and ferric ions Fe3+ (oxidised). The strength of partially oxidised sample is known. The solution of partially oxidised FeSO4 of known strength is titrated against standard KMnO4 solution to determine the molarity and strength of the unoxidised ferrous sulphate. From this the percentage oxidation of the sample can be calculated.
Indicator
KMnO4 is a self-indicator.
End Point
Colourless to permanent pink (KMnO4 in burette).
Procedure
- Weigh exactly 3.50 g of the given sample of ferrous sulphate on a watch glass and dissolve in water to prepare exactly 250 ml of solution using a 250 ml measuring flask. Rinse and fill the pipette with prepared ferrous sulphate solution and pipette out 20.0 ml of it in a washed titration flask.
- Rinse and fill the burette with the M/100 KMnO4 solution.
- Add one test-tube (~ 20 ml) full of dilute sulphuric acid (- 2 M) to the solution in titration flask.
- Note the initial reading of the burette.
- Now add KMnO4 solution from the burette till a permanent light pink colour is imparted to the solution in the titration flask on addition of a last single drop of KMnO4 solution.
- Note the final reading of the burette.
- Repeat the above steps 4—5 times to get three concordant reading.
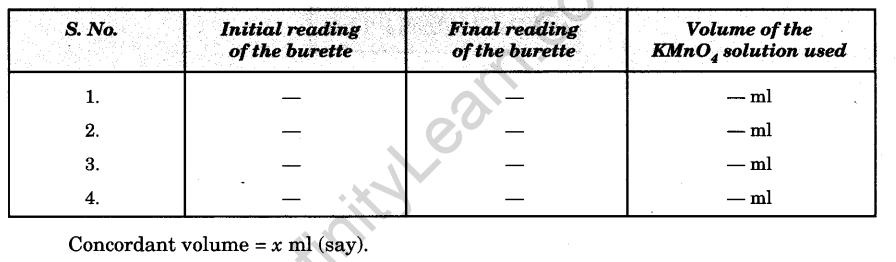
Observations
Weight of watch glass =……. g
Weight of watch glass + Mohr’s salt =…………..g
Weight of mixture = 3.50 g
Volume of solution prepared = 250 ml
Molarity of KMnO4 solution =M/100
Volume of oxalate solution taken for each titration = 20.0 ml.
Calculations
Molarity of the standard KMnO4 solution = M/100