Table of Contents
Very Short Answer Questions
Question 1.
Name some common metals.
Answer:
Copper, aluminium, iron, silver, gold, etc.
Question 2.
Name some common non-metals.
Answer:
Hydrogen, oxygen, carbon, sulphur, phosphorus, etc.
Question 3.
Give examples of metalloids.
Answer:
Antimony, arsenic, silicon, boron, etc.
Question 4.
Name the property due to which metals shine.
Answer:
Lustre
Question 5.
Name two metals which are soft enough to be cut.
Answer:
Potassium and sodium
Question 6.
Name the property due to which metals can be beaten into thin sheets.
Answer:
Malleability
Question 7.
Which non-metal does conduct heat and electricity?
Answer:
Carbon
Question 8.
Name the property due to which metals can be drawn into wires.
Answer:
Ductility
Question 9.
Name the metal and non-metal which occur in liquid state.
Answer:
Mercury (metal), bromine (non-metal).
Question 10.
Due to which property a bell rings?
Answer:
Sonority
Question 11.
Generally non-metals are non-lustrous. Name one non-metal which is lustrous.
Answer:
Iodine
Question 12.
State the property of non-metals due to which phosphorus is kept in water.
Answer:
Non-metals do not react with water.
Question 13.
Why some metals displace other metals from their solution?
Answer:
Because of being more reactive than the metals which they displace.
Question 14.
Which metal is used for wrapping food items?
Answer:
Aluminium
Question 15.
Which metal is more reactive: Iron or zinc?
Answer:
Zinc
Question 16.
Which metal is less reactive: Copper or zinc?
Answer:
Copper
Question 17.
Name one metal which does not react with dilute hydrochloric acid.
Answer:
Copper
Question 18.
Whose oxides are basic in nature: Metal or non-metal?
Answer:
Metal
Question 19.
Whose oxides are acidic: Metal or non-metal?
Answer:
Non-metal
Question 20.
Name two metals which do not react with oxygen even at high temperature.
Answer:
Gold and silver
Question 21.
Classify the following into metals and non-metals:
Copper, iron, graphite, sulphur, aluminium, oxygen
Answer:
Metals: Copper, iron, aluminium
Non-metals: Graphite, sulphur, oxygen
Question 22.
Name two physical properties of metals.
Answer:
Malleability and sonority
Question 23.
What happens when metals react with oxygen?
Answer:
Metal oxides are formed.
Question 24.
What happens when magnesium is burnt in air?
Answer:
Magnesium burns with a white dazzling flame and a white powdery magnesium oxide is formed.
Question 25.
What happens when metals react with water?
Answer:
Metals produce their hydroxides or oxides and hydrogen.
Materials: Metals and Non-Metals Class 8 Extra Questions Short Answer Questions
Question 1.
What is a metal?
Answer:
Substances having characteristic properties like malleability, ductility, sonority, conductivity, lustre, – and solidness are called metals. For example, aluminium, copper, zinc, iron, etc.
Question 2.
What are non-metals?
Answer:
Substances which are soft and dull, i.e., non-lustrous, non-sonorous, non-ductile, non-malleable and poor conductor of heat and electricity are called non-metals. For example, oxygen, hydrogen, sulphur, etc.
Question 3.
Mention the physical properties of metals.
Answer:
Physical properties of metals are:
- Malleable
- Lustre
- Sonorous
- Ductile
- Solid
- Good conductor of heat and electricity
Question 4.
What are the physical properties of non-metals?
Answer:
The physical properties of non-metals are:
- Non-malleable
- Non-sonorous
- Non-lustrous, i.e., dull in appearance
- Non-ductile
- Poor conductor of heat and electricity
Question 5.
Explain the term ‘malleability’ with suitable examples.
Answer:
Malleability is the property of metals due to which they can be beaten into thin sheets. For example, if we beat or hammer any metal like aluminium, zinc, iron, copper, etc., it become longer and larger but does not break. Thin sheets can be obtained by this process.
Question 6.
What is ductility? Explain with examples.
Answer:
Ductility is one of the properties of metals due to which they can be drawn into wires. For example, aluminium and copper are drawn into wires and used for electrical and different purposes.
Question 7.
Why aluminium is used for wrapping of food items?
Answer:
Aluminium is a metal and hence possesses malleability property. It can be beaten into thin sheets and can be folded into any shape. It is cheaper than other malleable metals and does not react with food items. That is why it is used as wrapping materials for food items.
Question 8.
Why metals are used in ringing bells?
Answer:
Metals have sonority. Due to this property, they can produce ringing sounds. That is why metals are used in ringing bells.
Question 9.
What are the differences between metals and non-metals? Explain on the basis of their physical properties.
Answer:
- Metals are malleable and give thin sheets after hammering whereas non-metals are brittle and give no sheets.
- Metals are ductile and can be drawn into wires whereas non-metals are non-ductile and can’t be drawn into wires.
- Metals are sonorous and used in ringing bells whereas non-metals are non-sonorous and cannot be used in ringing bells.
- Metals are good conductors of heat and electricity while non-metals are poor conductors.
Question 10.
What happen when a copper vessel is exposed to moist air for long? Also write the equation.
Answer:
When a copper vessel is exposed to moist air for long, it acquires a dull green coating. This green material is a mixture of copper hydroxide [Cu(OH)2] and copper carbonate (CuC03). The reaction is as follows:
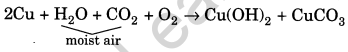
Question 11.
What happens when a magnesium ribbon is heated in presence of air?
Answer:
When a magnesium ribbon is heated in presence of air, it burns with a white dazzling flame and a white powdery magnesium oxide is formed.
![]()
Question 12.
How do metals and non-metals react with water?
Answer:
Metals produce their hydroxides or oxides and hydrogen when they react with water. Sodium and potassium react with cold water along with the production of a large amount of heat. Magnesium react with boiling water and iron with steam. Gold, silver and platinum do not react with water. Non-metals do not react with water.
Question 13.
With the help of equations, explain the reaction of non-metals with oxygen.
Answer:
Non-metals react with oxygen to form acidic oxides. But most of the non-metals reacts with oxygen on ignition. The equations follow as:
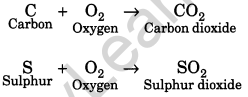
Question 14.
How do metals and non-metals react with acids?
Answer:
Metals react with acids to form respective salts along with evolution of hydrogen gas that burns with a pop sound. The equation are as follows:
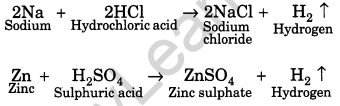
There are some metals like copper, silver, gold and platinum that do not liberate hydrogen with acids. Generally, non-metals do not react with acids.
Question 15.
How do metals and non-metals react with bases?
Answer:
Most of the metals do not react with bases. However, some metals like aluminium, lead and zinc react with strong bases like sodium hydroxide (NaOH) to make complex salts and hydrogen.
Generally, non-metals do not react with bases. Sometimes some complex reactions take place between non-metals and bases.
Question 16.
What is a displacement reaction? Give one example.
Answer:
A chemical reaction in which a more reactive metal displaces a less reactive metal is called displacement , reaction. For example, when zinc (Zn) reacts with copper sulphate (CuS04), zinc replaces copper being it more reactive than copper. The equation is
Zn + CuSO4 → ZnSO4 + Cu
Materials: Metals and Non-Metals Class 8 Extra Questions Long Answer Questions
Question 1.
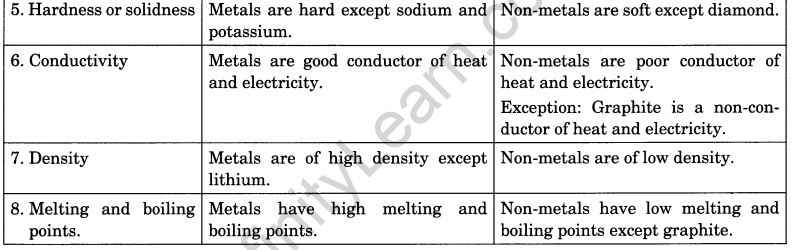
Distinguish between metals and non-metals on the basis of their physical properties.
or
Compare the physical properties of metals and non-metals.
Answer:
Difference between metals and non-metals on the basis of their physical properties.
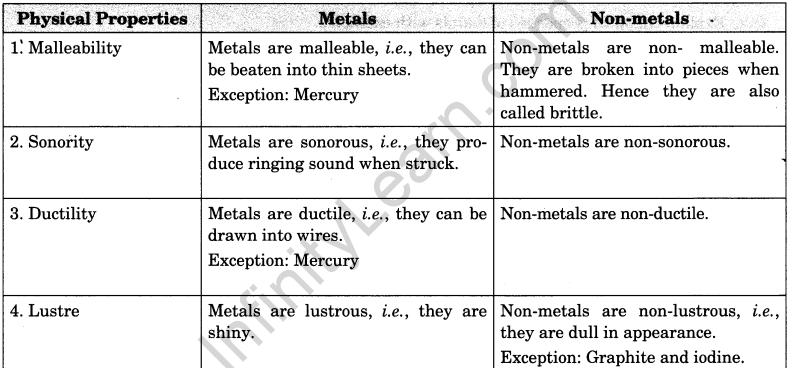
Question 2.
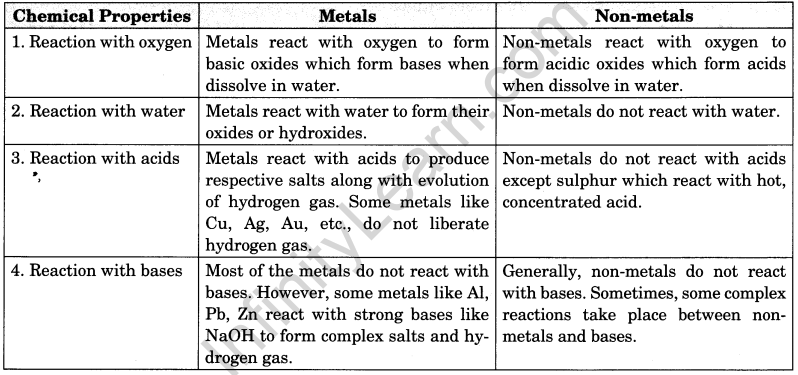
Distinguish between metals and non-metals on the basis of their chemical properties.
or
Compare between metals and non-metals on the basis of their chemical properties.
Answer:
Difference between metals and non-metals on the basis of their chemical properties.
Question 3.
Explain chemical properties of metals with examples.
Answer:
(i) Reaction with oxygen or air: Metals react with oxygen to form basic oxides.
Some metals like potassium and sodium react vigorously with oxygen. For example,
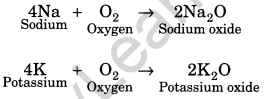
Some noble metals like gold, silver and platinum do not react with oxygen. Iron (Fe) and copper (Cu) get rusted when react in presence of oxygen and water (moist air).
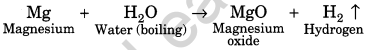
(ii) Reaction with water: Metals react with water to form their oxides or hydroxides. Gold, silver and platinum do not react with water. Some metals like sodium, potassium react vigorously with water. For example,
![]()
(iii) Reaction with acids: Metals react with acid to form their salts followed by evolution of hydrogen gas. For example,
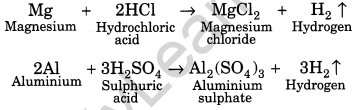
Some metals like gold, copper, silver, etc., do not liberate hydrogen gas with acids.
(iv) Reaction with bases: Most of the metals do not react with bases. However some metals like alu-minium, zinc and lead react with strong bases like sodium hydroxide to make complex salts and produce hydrogen.
(v) Displacement reactions: More reactive metals displace less reactive metals. For example,
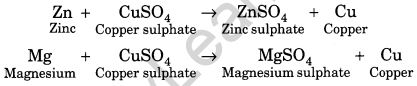
In the above reactions, zinc (Zn) and magnesium (Mg) are more reactive than copper (Cu), hence they replace copper from its solution.
Question 4.
Explain with suitable examples the chemical properties of non-metals.
Answer:
(i) Reaction with oxygen: Non-metals react with oxygen to form acidic oxides. But most of them do this on ignition. For example,
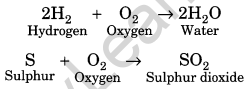
(ii)They form negative ions and are good oxidising agents.
(iii) Reaction with water: Non-metals do not react with water.
(iv) Reaction with acids and bases: Generally, non-metals do not react with acids and bases. How¬ever, sometimes some complex reactions take place between non-metals and bases.
Question 5.
What are main uses of metals?
or
How are metals useful to us?
Answer:
Metals are very useful to us in many ways. For example,
- Due to their thermal and electrical conductivity, metals are use to make utensils, cooking vessels, wires and appliances. For example, copper and aluminium are mainly used for these purposes.
- Metals like iron and steel are used in various tools, machinery, pipes, rods, sheets, doors, windows, construction works like bridges, roads, buildings, etc.,
- Aluminium is used as packaging and wrapping materials. It is also used in aircrafts and automobiles, etc.
- Metals like gold, silver and platinum are used to make jewellery and other decorating items.
- Zinc is used in galvanisation and dry cell and chromium in electroplating.
- Lead is used in making electrodes and batteries.
Question 6.
What are the main uses of non-metals?
or
How are non-metals useful to us?
Answer:
Like metals, non-metals also play an important role in our lives. They help us in many ways. For example,
- We breathe oxygen which is the basis of life of all living things including human beings. Without it, no living beings can exist alive on this earth.
- CO2 which is a non-metal oxide is essential for plants to carry out photosynthesis.
- Non-metals like nitrogen and phosphorus are used in fertilisers for better yield of plant. Phosphorus is used in manufacturing of matchsticks and fireworks.
- Non-metal like iodine is used in the purple coloured solution applied on wounds. Sulphur is also used in preparing skin medicines and making ointment due to its fungicidal properties.
- Non-metal like chlorine is used in water purification process. Due to its bleaching properties it is used to make bleaching powder.
- Carbon, a non-metal, is used in most of the fuels.
Question 7.
What is reactivity series? Suggest an activity to arrange sodium, magnesium and copper in the order of their decreasing reactivity.
Answer:
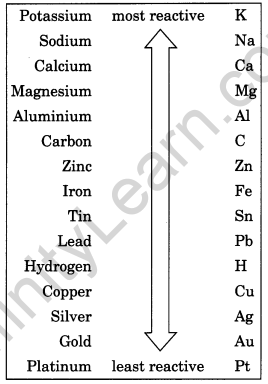
Reactivity series is an arrangement of metals in decreasing order of their reactivity from highest to lowest. The metals occupying the higher positions in the activity series are more reactive in displacing the other metals lying below it from the solutions of their salts.
The activity series is a useful guide for predicting the products of metal displacement reactions. For example, placing a strip of zinc metal in a copper (II) sulphate solution will produce metallic copper and zinc sulphate, since zinc is above copper on the series.
A strip of copper placed into a zinc sulphate solution will not produce an appreciable reaction, because copper is below zinc on the series and can’t displace zinc ions from solution.
Activity to arrange sodium, magnesium and copper in the order of their decreasing reactivity:
- Take a pinch of sodium with a forceps and place in a beaker containing water. You will notice that sodium reacts vigorously.
2Na(s) + 2H2O(aq) → 2NaOH(aq) + H2(g) - Take a small piece of magnesium ribbon and add warm water to it. Magnesium reacts with warm water to form magnesium oxide and hydrogen gas. Magnesium reacts very slowly with cold water.
Mg(s) + 2H2O(aq) → Mg(OH)2(aq) + H2(g) - Take small pieces of copper turnings and add warm water to it. It doesn’t react with warm water also.
Cu(s) + 2H2O(aq) → No reaction
Hence, increasing order of reactivity is Na > Mg > Cu.
Materials: Metals and Non-Metals Class 8 Extra Questions Higher Order Thinking Skills
Question 1.
Why does calcium float in water?
Answer:
The hydrogen gas formed on adding calcium to water sticks to the surface of calcium solid and make it float in water.
Question 2.
Zinc sulphate forms a colourless solution in water. Will you observe any colour on adding copper turn-ing in it?
Answer:
No, because copper is less reactive than zinc and will not be able to displace zinc from its salt solution.
Question 3.
A doctor prescribed a tablet to a patient suffering from iron deficiency. The tablet does not look like iron. Explain.
Answer:
The tablet is not made of iron metal, instead it contains a salt of iron.
Question 4.
Ram stored copper sulphate solution in a container made of iron. He observed certain changes after few hours. Can you tell what changes did he observed?
Answer:
Blue colour solution of copper sulphate has changed to green colour of ferrous sulphate. The iron container was found to be corroded from many places. A red powdery deposit of copper sulphate was found on the iron container.
Question 5.
Imagine that gold is reactive like copper. Will it be still wanted? Why or why not?
Answer:
If gold becomes reactive like copper then its use in ornaments will decline. This is because due to its increase reactivity it will loose its shine frequently which in turn will reduce its demand.
Materials: Metals and Non-Metals Class 8 Extra Questions Value-Based Questions
Question 1.
Gold is a very precious metal. Pure gold is very soft and is thus not suitable for making jewellery. It is alloyed with either silver or copper to make it hard. But some jewellers mix a large quantity of copper and silver in gold to earn more profit.
(a) What precautions should you take while purchasing gold jewellery?
(b) What standard you must see on gold ornaments?
(c) What value of shopkeeper’s are shown here?
Answer:
(a) We must see the carat of gold jewellery, current price and BIS hallmark on it.
(b) BIS hallmark.
(c) Some shopkeeper’s are greedy, mean, cheater and money minder.
Question 2.
Mercury is largely used in thermometers to measure the temperature. It is a very dangerous metal as its density is very high. If it get into the food chain, it leads to mercury poisoning.
(a) What two precautions you must take while handling equipments containing mercury?
(b) Why mercury is used in thermometers?
(c) Can you suggest other alternatives to mercury thermometers?
Answer:
(a)
- We must handle the equipments carefully and firmly.
- If there is a mercury spill, we must leave the area immediately and inform our parents or teachers.
(b) Mercury is a good conductor of heat. Hence, the slightest change in temperature is potable when it is used in a thermometer.
(c) Digital thermometer and spirit thermometer.
Activities and Projects
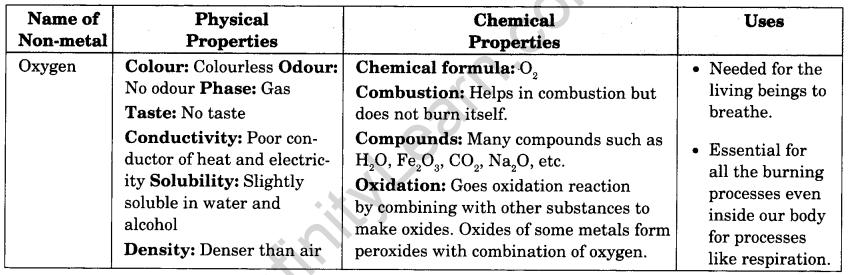
Question 1.
Prepare Index Cards for any four metals and four non-metals. The card should have information like name of metal/non-metal; its physical properties, chemical properties and its uses.
Answer:
An index card is used for recording and storing small sizes of discrete data. It consists of heavy paper cut to a standard size.
Here, one example of non-metal is done for you. The rests you should try to do yourself.
Question 2.
Visit a blacksmith and observe how metals are moulded.
Answer:
Do it yourself.
Question 3.
Suggest an experiment to compare the conductivity of electricity by iron, copper, alumini¬um and zinc. Perform the experiment and prepare a short report on the results.
Answer:
You can do this yourself with the help of electric tester. The process is explained here for your help.
Take an electric tester and test the conductivity of the given metals separately with the wires of same length and thickness. Observe the brightness of the lighting bulb. Now compare the conductivity of all metals according to their brightness as you observed. Finally prepare a short report based on your findings.
Question 4.
Find out the locations of the deposits of iron, aluminium and zinc in India. Mark these in an outline map of India. In which form are the deposits found? Discuss in the class.
Answer:
The locations of the deposits of these metals may be shown on the map of India. You are advised to practice it at home. Also have a discussion on this in the class.
Question 5.
Discuss with your parents/neighbours/goldsmiths why gold is preferred for making jewellery.
Answer:
Gold is a special metal in terms of ductility and malleability. It can be drawn into wires thinner that a hair or beaten into thin sheets. That is why it is prepared for making jewellery.
Question 6.
Visit the following websites and enjoy the quiz on metals and non-metals:
- chemistry.about.com/library/weekly/bl050303a.htm
- chemistry.about.com/od/testsquizzes/Chemistry_Tests_Quizzes.htm
- www.gcsescience.com/q/qusemet.html
- www.corrosionsource.com/handbook/periodic/metals.htm
Answer:
You, can do it yourself for more knowledge about metals and non-metals.
I. Multiple Choice Questions (MCQs)
Choose the correct option.
Question 1.
Metals are
(a) shiny
(b) hard
(c) sonorous
(d) all of these
Question 2.
Non-metals are
(a) non-ductile
(b) non-sonorous
(c) non-malleable
(d) all of these
Question 3.
Which of the following is a non-metal?
(a) Aluminium
(b) Oxygen
(c) Iron
(d) Siolver
Question 4.
Metalloids possess the properties of
(a) metals
(b) non-metals
(c) both metals and non-metals
(d) none of these
Question 5.
The most reactive metal is
(a) copper
(b) zinc
(c) Potassium
(d) gold
Question 6.
Non-metals are
(a) generally gases
(b) generally liquids
(c) generally solids
(d) generally solid and gasses
Question 7.
Which of the following metal is stored in kerosene?
(a) Sodium
(b) Magnesium
(c) Phosphorus
(d) Zinc
Question 8.
Metal oxides are
(a) neutral
(b) basic
(c) acidic
(d) all of these
Question 9.
The non-metal which is liquid at room temperature is
(a) bromine
(b) chlorine
(c) iodine
(d) carbon
Question 10.
Which substance is used for making pencil lead?
(a) Sulphur
(b) Silicon
(c) Graphite
(d) Aluminium
Question 11.
Which non-metal is used in making glass?
(a) Graphite
(b) Sulphur
(c) Silica
(d) None of these
Question 12.
Which metal is used in wrapping materials?
(a) Aluminium
(b) Zinc
(c) Copper
(d) None of these
Question 13.
The metal found in liquid state is
(a) mercury
(b) silver
(c) calcium
(d) sodium
Question 14.
The most ductile metal is
(a) silver
(b) gold
(c) copper
(d) aluminium
Question 15.
Non-metals
(a) react with water
(b) do not react with water
(c) both (a) and (b)
(d) none of these
Question 16.
Which of the following metals are soft enough to be even cut with a knife?
(a) Sodium
(b) Potassium
(c) Lithium
(d) All of these
Question 17.
Which is the hardest substance?
(a) Gold
(b) Diamond
(c) Aluminium
(d) None of these
Question 18.
Metals react with acids to produce respective salts with evolution of
(a) hydrogen gas
(b) oxygen gas
(c) CO2 gas
(d) none of these
Question 19.
In displacement reactions
(a) a more reactive metal displaces a less reactive metal.
(b) a less reactive metal displaces a more reactive metal.
(c) both (a) and (b)
(d) none of these
Question 20.
Which of the following non-metals are used in fertilisers?
(a) Nitrogen
(b) Phosphorus
(c) Both (a) and
(d) None of these
Answer:
1. (d)
2. (d)
3. (b)
4. (c)
5. (c)
6. (d)
7. (a)
8. (b)
9. (a)
10. (c)
11. (c)
12. (a)
13. (a)
14. (b)
15. (b)
16. (d)
17. (b)
18. (a)
19. (a)
20. (c)
II. Fill in the Blanks
Fill in the blanks with suitable word/s.
1. Metals are _____ of heat and _____.
2. Iodine is a _____ having lustre.
3. _____ and _____ are kept in kerosene to avoid explosion.
4. Non-metal oxides are _____ in nature.
5. _____ is more reactive than copper.
6. Sulphur forms _____ oxides.
7. Magnesium forms _____ oxides.
8. _____ is less reactive than iron.
9. All metals are hard except _____ and _____.
10. Metals have generally _____ melting and boiling points.
11. _____ are used in medicines as antiseptic.
12. The only liquid metal is _____.
13. _____ is non-metal used in breathing by all living beings.
14. The metal which produces hydrogen gas on reaction with dilute hydrochloric acid as well as sodium hydroxide solution is _____.
15. _____ reacts with cold water vigorously.
Answer:
1. good conductors, electricity
2. non-metal
3. Sodium, potassium
4. acidic
5. Zinc
6. acidic
7. basic
8. Copper
9. sodium, potassium
10. high
11. Non-metals
12. mercury
13. Oxygen
14. aluminium
15. Sodium
III. Match the following
Match the items given in column I suitably with those given in column II.
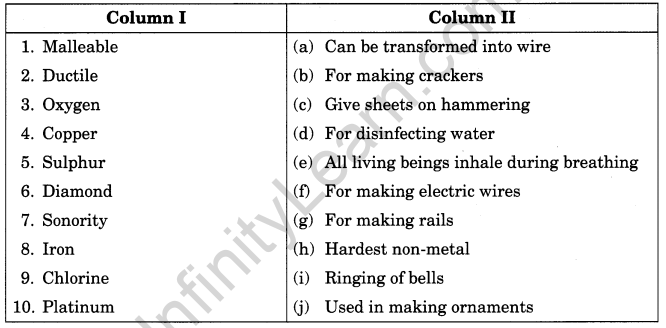
Answer:
1. (c)
2. (a)
3. (e)
4. (f)
5. (b)
6. (h)
7. (i)
8. (g)
9. (d)
10. (j)
IV. True or False
State whether the given statements are true or false.
1. Metals are non-sonorous.
2. Metals react with water.
3. Non-metals cannot be converted into wires.
4. The only liquid metal is bromine.
5. Sodium and potassium do not react vigorously with water and oxygen.
6. Basic solution turns red litmus into blue.
7. Graphite is a good conductor of electricity.
8. Generally, metallic oxides are basic and non-metallic oxides are acidic in nature.
9. Chlorine is not a non-metal.
10. Phosphorus is kept in water.
11. All metals exist in solid form at room temperature.
12. Rust formed on iron object is acidic in nature.
13. Aluminium is more reactive than copper.
14. Non-metals react with water to form a gas which burns with a ‘pop’ sound.
15. All the gases are non-metals.
Answer:
1. False
2. True
3. True
4. False
5. False
6. True
7. True
8. True
9. False
10. True
11. False
12. False
13. True
14. False
15. True



