
Courses

By Shailendra Singh
|
Updated on 28 Feb 2025, 15:49 IST
Carbon, symbolized as 'C' with an atomic number of 6, is one of the most versatile and vital elements found in nature. Carbon's ability to form bonds in various ways gives rise to different structural forms known as allotropes. These allotropes exhibit distinct physical and chemical properties despite being composed solely of carbon atoms. Carbon allotropes can broadly be categorized into crystalline and amorphous forms.
Carbon, with an atomic number of 6, and a symbol of ‘C’ in the periodic table, is one of the most powerful elements we encounter. Carbon is an example of an element that exhibits allotropy. Carbon allotropes can be amorphous or crystalline in nature (Diamond, Graphite).
Carbon is one of the few elements that can have different allotropic forms due to its capacity to have variable oxidation states or coordination numbers. Another important component is carbon’s capacity to catenate. As a result, it leads to the development of diverse carbon allotropes.
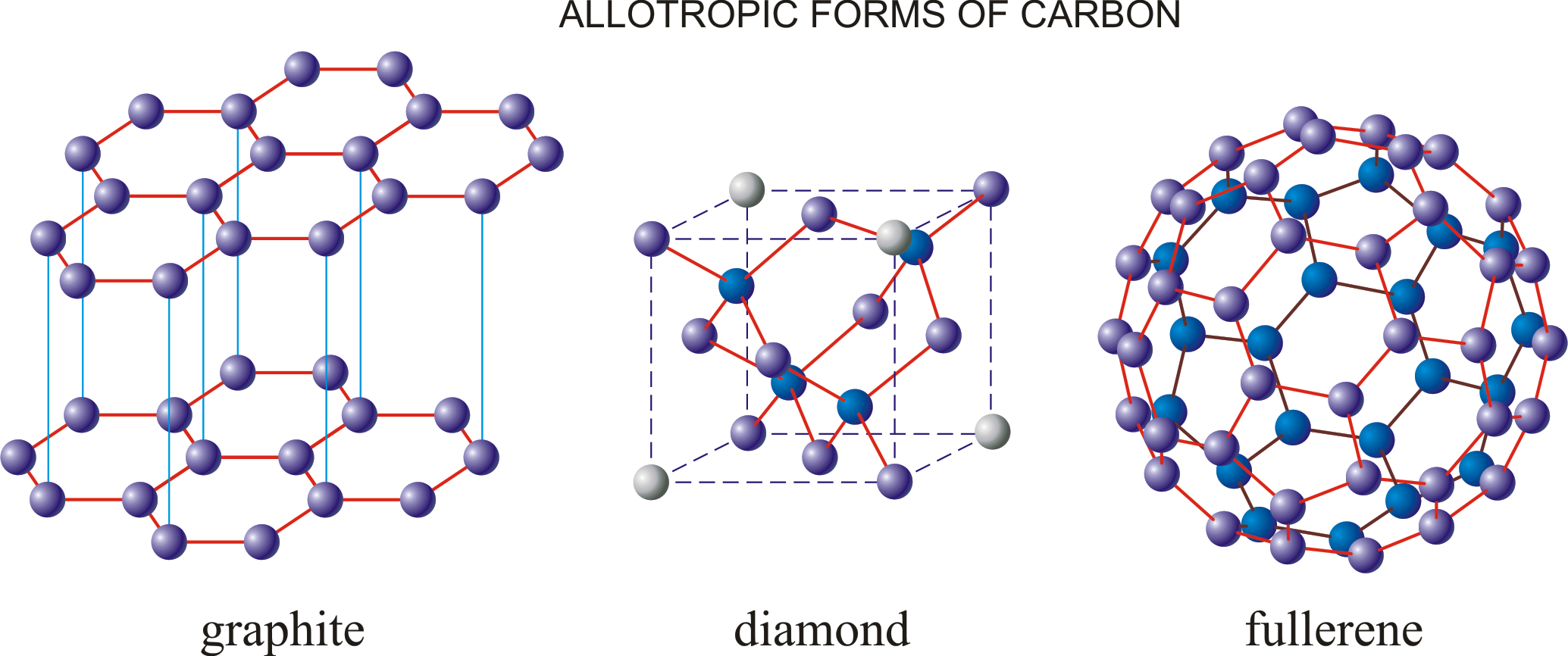
Crystalline allotropes of carbon include diamond, graphite, fullerenes, carbon nanotubes, and lonsdaleite. These forms exhibit organized, repeating patterns of carbon atoms, which account for their unique properties.
| Property | Diamond | Graphite |
| Hardness | Extremely hard | Soft |
| Electrical Conductivity | Poor | Good |
| Thermal Conductivity | High | Moderate |
| Structure | Tetrahedral lattice | Layered hexagonal |
Amorphous forms of carbon lack a definite crystalline structure and include charcoal, carbon black, and coal.
| Allotrope | Structure | Electrical Conductivity | Thermal Conductivity | Key Features |
| Diamond | Tetrahedral lattice | Poor | High | Hardest natural material. |
| Graphite | Layered hexagonal | Good | Moderate | Excellent lubricant. |
| Fullerene (C₆₀) | Spherical cage | Moderate | Moderate | Can act as an electron acceptor. |
| Carbon Nanotubes | Cylindrical graphene roll | Excellent | Excellent | High tensile strength, nanoelectronics. |
| Q-Carbon | Ferromagnetic crystalline | Unknown | High | Brighter and harder than diamond. |
| Allotrope | Applications |
| Diamond | Cutting tools, abrasives, heat sinks, and jewelry. |
| Graphite | Pencils, lubricants, electrodes, and high-temperature crucibles. |
| Fullerene | Drug delivery, superconductors, and nanomedicine. |
| Carbon Nanotubes | Nanoelectronics, composite materials, and energy storage. |
| Amorphous Carbon | Water filtration, inks, and fuels. |
Carbon's diverse allotropes, from the hardness of diamond to the softness of graphite, illustrate its incredible versatility. These forms have unique structures and properties, making them integral to numerous applications across industries such as electronics, medicine, and energy. Understanding the characteristics and uses of carbon allotropes opens up endless possibilities for scientific advancements.


Fullerene is the purest form of carbon because it lacks the gleaming edges and surface bonds that attract other atoms found in graphite and diamond.
The condensation of Cn small molecules produces a sooty substance when graphite is heated in an electric arc in an inert atmosphere such as helium or argon, culminating in fullerene. The C60 and C70 fullerenes contained in sooty material are separated from the fullerenes found in soot by extraction with benzene or toluene followed by chromatography over alumina.