
Courses

By Shailendra Singh
|
Updated on 23 Jan 2025, 17:47 IST
Boranes are boron hydrides with BH groups occupying the apices of electron-deficient clusters. Because boron has more valence orbitals than valence electrons, the term “electron-deficient” was coined. As a result of this imbalance, the clusters are held together by extensive multicentered bondings.
The relationships between cage structures and the number of cage electrons are discussed for boranes, as well as their extension to heteroboranes in which one or more BH units are replaced by isolobal main group or transition metal moieties. General synthetic methods and heteroboranes’ reactivities are also discussed. Boranes are compounds with the formula BxHy and their related anions.
There are numerous boranes of this type. Those with 1 to 12 boron atoms are the most common. Although they have few practical applications, boranes have structures and bonding patterns that differ significantly from those seen in hydrocarbons.
Boranes-hydrocarbon hybrids, known as carboranes, are also well developed. Boron hydrides are binary compounds of boron and hydrogen. Boranes are the name given to these boron hydrides. In general, boron hydrides are highly reactive and volatile substances.
Their low boiling points are a result of their low molecular weights and weak intermolecular forces. Their reactivities are thermodynamic in nature, as these compounds are highly unstable when their boron-boron and boron-hydrogen bonds are replaced by bonds to more electronegative elements.
Alfred Stock, a German chemist, was the first to systematically synthesise and characterise boron hydrides between 1912 and 1937. He named them boranes after the alkanes (saturated hydrocarbons), which are hydrides of carbon (C), which is boron’s neighbour in the periodic table.
Stock developed high-vacuum methods and apparatus for studying lighter boranes because they were volatile, sensitive to air and moisture, and toxic. Hermann, I began work on boranes in the United States in 1931. Diborane is the most basic boron hydride known. It is made by combining boron trifluoride and LiAlH4 in diethyl ether.
4BF3 + 3 LiAlH4 → 2B2H6 + 3LiF + 3AlF3

The oxidation of sodium borohydride with iodine is a convenient laboratory method for the preparation of diborane.
2NaBH4 + I2 → B2H6 + 2NaI + H2
Diborane is synthesised on a large scale by reacting BF3 with sodium hydride. Diborane is a colourless, highly toxic gas with a boiling point of 180 degrees Celsius. When exposed to air, diborane spontaneously catches fire. It burns in oxygen, releasing massive amounts of energy.
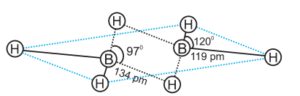
The four-terminal hydrogen atoms and two boron atoms are all in the same plane. There are two bridging hydrogen atoms above and below this plane. The four-terminal B-H bonds are regular two-center–two-electron bonds, whereas the two bridge (B-H-B) bonds are unique and can be described as three-center–two-electron bonds.
Boron also produces a number of hydridoborates, the most important of which is the tetrahedral [BH4] – ion. Several metal tetrahydridoborates are known. The reaction of metal hydrides with B2H6 in diethyl ether produces lithium and sodium tetrahydridoborates, also known as borohydrides.

Boron family members react with halogens to form tri-chlorides, bromides, and iodides. These halides have covalent bonds and are hydrolyzed in water. Because of the electron deficiency, these trihalides are strong Lewis acids; as we move from boron to thallium, the metallic character increases. Electronegativity of the elements decreases first down the group from B to Al, then increases marginally due to differences in atomic size.
The periodic table’s group 13 elements are the first group in the p-block. The boron family refers to all of the elements in group 13. The periodic table is divided into four sections: s, p, d, and f. This segregation is based on the valence electron; if the valence electron is on the p subshell, it enters the p-block, and so on.
B is a member of Group 13. It produces the electron-deficient hydride B2H6. To complete its octet, the central B atom has 6 valence electrons and can accept 2 electrons (a lone pair from a base). As a result, boron forms hydride, which is Lewis acid. N is a member of Group 15. It produces the electron-rich hydride NH3. The central N atom contains a single electron pair that can be donated to a suitable Lewis acid. As a result, nitrogen forms hydride, which is a Lewis base.
Group 13 hydrides are chemical compounds with 13-hydrogen bonds (elements of group 13: boron, aluminium, gallium, indium, thallium). Boranes have a wide range of covalent cluster chemistry, but heavier group 13 hydrides do not. Regardless of their formulae, they tend to form polymers.
Alane is a powerful reducing agent composed of octahedrally coordinated aluminium atoms. Gallane is even more difficult to synthesise and decomposes at room temperature to gallium and hydrogen.
Indigane and thallane are too unstable to exist for an extended period of time if not coordinated.
Lithium borohydride (LiBH4) is a borohydride that is used as a reducing agent for esters in organic synthesis. Although less common than sodium borohydride, lithium salt has some advantages, including being a stronger reducing agent and being highly soluble in ethers, while remaining safer to handle than lithium aluminium hydride.
Lithium borohydride is an all-purpose reducing agent that is commonly used to reduce aldehydes, ketones esters, lactones, and epoxides. It catalyses alkene hydroboration. It is also used to make other borohydrides, such as aluminium borohydride. Because of its higher chemoselectivity compared to other popular reducing agents such as lithium aluminium hydride, the use of lithium borohydride is particularly advantageous in some preparations.
Lithium borohydride, for example, reduces esters, nitriles, lactones, primary amides, and epoxides while sparing nitro groups, carbamic acids, alkyl halides, and secondary/tertiary amides.
Covalent hydrides are formed by elements in Group 13. The majority of these are electron-deficient hydrides. They function as Lewis acids.
Hydrides in group 13 (e.g., BH3, AlH3, etc.) have fewer electrons available to form normal covalent bonds and are thus referred to as electron-deficient hydrides. To compensate for this deficiency, these hydrides are commonly found in polymeric forms such as B2H6, B4H10, (AlH3)n, and so on. They are all Lewis bases or electron donors.
Group 13 hydrides are Lewis acids, while group 15 hydrides are Lewis bases. Because they form electron-deficient compounds, all of the elements in group 13 are hydrides that act as Lewis acids.