
Courses

By Shailendra Singh
|
Updated on 28 May 2025, 18:29 IST
Proteins fold into different three-dimensional structures in the vast majority of cases. A protein’s natural conformation is the shape into which it folds spontaneously. Because of the chemical properties of their amino acids, many proteins can fold on their own, while others require the aid of molecular chaperones to fold in their natural forms.
A protein structure is characterized as a polymer of amino acids linked together by peptide bonds. Protein structures are formed by the condensation of amino acids, which create peptide bonds. The primary structure of a protein is its sequence of amino acids. The dihedral angles of the peptide bonds determine the secondary structure, while the folding of protein chains in space determines the tertiary structure.
The formation of a quaternary structure occurs when folded polypeptide molecules bind to complex functional proteins. Proteins fold into different three-dimensional structures in the vast majority of cases. A protein’s natural conformation is the shape into which it folds spontaneously. Because of the chemical properties of their amino acids, many proteins can fold on their own, while others require the aid of molecular chaperones to fold in their natural forms.
Also Check: Denaturation of Proteins and its Causes
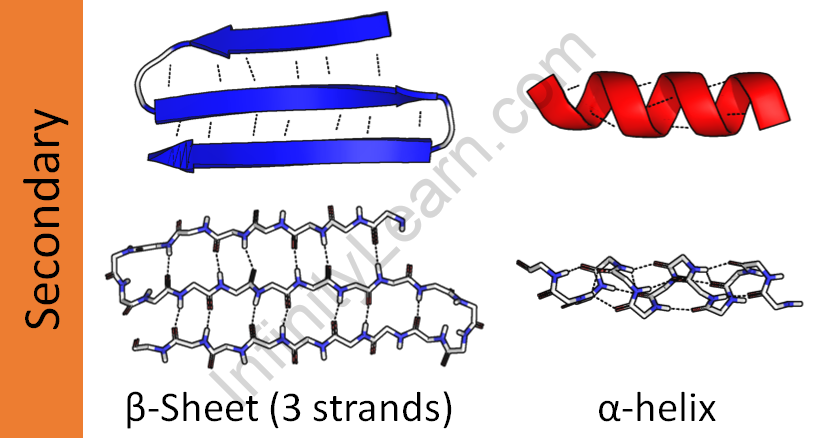
The interaction between the repeating peptide unit’s hydrogen bond donor and acceptor residues is referred to as a secondary structure. Linus Pauling, an American scientist, predicted the two most essential secondary structures of proteins, the alpha helix, and the beta-sheet, in the early 1950s.
Pauling and his colleagues discovered that, among other things, the folding of peptide chains should retain the bond angles and planar shape of the peptide bond, as well as keep atoms from coming together so tightly that they repelled each other via van der Waal’s interactions.
Finally, Pauling anticipated that hydrogen bonds must be capable of stabilizing peptide backbone folding. The conditions are met by two secondary structures: the alpha helix and the beta-pleated sheet. Pauling’s prognosis was right. The majority of identified secondary structures observed in proteins are of one of two types.
However, parts of the protein chain can develop their own local fold, which is simpler and typically takes the form of a spiral, an expanded shape, or a loop. These local folds are known as secondary elements, and they contribute to the protein’s secondary structure.


When amino acids are linked together by peptide bonds, they produce a polypeptide, which is another term for protein. The polypeptide subsequently folds into a specific shape depending on the interactions (strained lines) between its amino acid side chains.
Protein folding is the process through which a protein structure takes on its functional shape or conformation. Protein molecules are made up of heterogeneous unbranched amino acid chains. They may carry out their biological purpose by coiling and folding into a certain three-dimensional shape.
The primary structure of a protein is the linear sequence of amino acids within it. Proteins are made up of a sequence of only twenty amino acids, each with a unique side chain. Amino acid side chains are chemically different.