
Courses

By Shailendra Singh
|
Updated on 28 Apr 2025, 12:34 IST
Benzene is a chemical that, at normal temperature, is a colorless or light yellow liquid. It has a pleasant odor and is quite combustible. It evaporates fast into the air. Its vapor is heavier than air and can sink to the ground in low-lying locations. Benzene dissolves slowly in water and floats on top of it.
Benzene Formula- It has the chemical formula C6H6 and is an aromatic organic molecule. In nature, there is an abundance of benzene. There is no need to synthesize it because it is a natural component of gas emissions from volcanic or forest fires, and it is also present in gasoline, crude oil, and cigarette smoke.
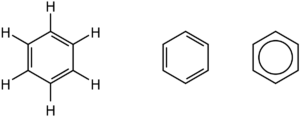
The benzene molecule is made up of six carbon atoms bonded together in a planar ring, each having one hydrogen atom connected to it. Benzene is classified as a hydrocarbon since it exclusively includes carbon and hydrogen atoms. Benzene is classified as an aromatic hydrocarbon due to the cyclic continuous pi bonds that exist between the carbon atoms.
The term “benzene” is derived from “gum benzoin” (benzoin resin), an aromatic resin known to European pharmacists and perfumers as a product of Southeast Asia since the 16th century.
Also Check: Electrophilic Substitution of Benzene
Benzene Structure has cyclic hydrocarbon (C6H6), which means that each carbon atom in benzene is organized in a six-membered ring and is connected to just one hydrogen atom. According to molecular orbital theory, the development of three delocalized – orbitals spanning all six carbon atoms occurs in the benzene ring, however, valence bond theory predicts two stable resonance configurations for the ring.
Also Check: Benzene Hexachloride
The oscillating double bonds in the benzene ring are described using valence bond theory’s resonance structures. The benzene ring’s carbon atoms are all sp2 hybridized. One of one atom’s two sp2 hybridized orbitals coincides with the sp2orbital of a nearby carbon atom, resulting in six C-C sigma bonds. Six C-H sigma bonds are formed when other left sp2 hybridized orbitals join with the s orbital of hydrogen. Carbon atoms’ remaining unhybridized p orbitals establish bonds with neighboring carbon atoms via lateral overlap. This explains how C1 – C2, C3 – C4, C5 – C6 bonds can develop in the same way as C2 – C3, C4 – C5, C6-C1 π bonds.

Because the C-C bonds generated in the ring are not exactly single or double, but rather of intermediate length, benzene is an aromatic compound. Aromatic compounds are classified into two types: benzenoids (those with a benzene ring) and non-benzenoids (those without a benzene ring) if they follow the Huckel rule.
According to the Huckel rule, an aromatic ring should contain the following properties:
Also Check: Substituted Benzene Compounds
Benzene is utilized in a variety of industrial processes, including the production of lubricants, plastics, rubbers, dyes, synthetic fibers, and so on. However, because benzene is poisonous and carcinogenic, it has few non-industrial uses. The various applications of Benzene are listed below:
Benzene is contained in almost all items used in the printing business. There are products that include this chemical and are specifically used for cleaning printing equipment, making it last longer and work better. Furthermore, benzene may be found in ink and a range of painting goods such as spray paints, sealers, lacquers, and stains. It maintains the paints’ liquid state.
Many individuals and corporations utilize benzene as a fuel because of its high octane rating and natural availability. It has been used as a gasoline additive to help fuel burn more effectively.
Benzene is widely utilized as a solvent in a variety of industrial, commercial, and research applications. Manufacturers employ benzene-containing goods as solvents at various stages of production, and it is used in the creation of chemical and plastic products. Resins, synthetic materials such as nylon, Styrofoam, and others are a few examples. Benzene is also used to make asphalt, which is utilized by roofing and paving industries.
Benzene is also utilized in the manufacture of tires and rubber, as well as in adhesives used to adhere shoe bottoms to shoes. Detergents, pesticides, insecticides, herbicides, and dyes are among the other chemical substances produced utilizing benzene.
Borazine (B3N3H6), also known as borazole, is inorganic benzene. Because the structure of borazine is similar to that of benzene, it is sometimes known as inorganic benzene. With benzene, it is also isoelectronic and isosteric. Both N and B in borazine are sp2 hybridized, much as carbon in benzene. Each N has a p-orbital that is perpendicular to the bonding orbitals and contains a single electron pair. Each B, on the other hand, has an empty p-orbitals that is likewise perpendicular to the plane of the ring. Thus, the bonding in borazine is dative, resulting from the lateral overlap of full N orbitals and empty B p-orbitals.
Borazine is made by combining diborane with ammonia at low temperatures to generate an additional product.
B2H6+2NH3→B2H6.2NH3
When this addition compound is heated to 473 degrees Celsius, it decomposes to yield the volatile chemical borazine.
3B2H6+2NH3→2B3N3H6+12H2
However, the chemical also carries the health concerns linked with benzene exposure. It has a number of side effects, including headaches, instability, convulsions, unconsciousness, and irritations. It can have both acute and chronic effects depending on the level of exposure. And this can happen whether Benzene is consumed by mouth, breathed through the air, or absorbed through the skin. People are often exposed to benzene when they fill up their automobiles with fuel, use benzene-containing household products, or drink polluted water.
When exposed to benzene, your bone marrow cells may stop producing red blood cells or your immune system's white blood cells may fail. There is a window of time after smelling benzene after a leak to take action or leave the area without harm, but prolonged exposure can be deadly.
Benzene is a common solvent that may be found in gasoline, automobile exhaust, and cigarette smoke. High-level benzene pollution has historically been ubiquitous, and because of its carcinogenic properties, benzene contamination has often been a major source of worry.
Benzene is an aromatic, colorless chemical molecule that is commonly utilized in manufacturing. It is also utilized as a binding and absorbing agent. It is utilized in the production of tires and rubber. It is utilized in the manufacture of cleaning agents for vehicle components due to its high solubility. The use of benzene aids in the removal of grease from hydraulic systems, brakes, and other car components. It is a common element in paints, spray paint, lacquers, dyes, stains, and other painting goods. The inclusion of benzene in paints keeps it liquid until it is utilized.