
Courses

By Shailendra Singh
|
Updated on 24 Jan 2025, 13:11 IST
In general, the electrostatic attraction of oppositely charged ions in a chemical molecule results in the formation of an ionic bond, also known as an electrovalent bond. This type of bond is formed when the valence (outermost) electrons of one atom are permanently transferred to another atom. The atom that loses electrons becomes a positively charged ion (cation), while the atom that acquires them becomes a negatively charged ion (anion). Ionic bonding is the process of forming ionic connections between two or more molecules. Ionic bonding creates ionic or electrovalent compounds between nonmetals and alkali/alkaline-earth metals.
The ionic crystalline solids of this type, the electrostatic forces of attraction between opposite charges and repulsion between comparable charges position the ions in such a way that each positively charged ion is surrounded by negative ions and vice versa. In a nutshell, the ions are arranged in such a way that the positive and negative charges alternate and balance one another, resulting in a charge of zero for the entire substance. In ionic crystals, the electrostatic forces are extremely strong. As a result, these compounds are often hard and non-volatile.
We can say that an ionic bond is a bond between a metal and a nonmetal that is responsible for holding oppositely charged ions together by a strong electrostatic attraction. An electrovalent bond is formed when electrons are transferred from the outermost shell of metal to the outermost shell of a nonmetal.
The octet rule is satisfied when ions exchange valence electrons in order to achieve electron configurations that are similar to those of noble gases. The octet rule asserts that an atom is most stable when its valence shell has eight electrons. Atoms with less than eight electrons in their valence shell tend to follow the duet rule and have two electrons in their valence shell. Ions are more stable when they follow the duet or octet rules.
One atom, usually a metal, transfers one or more valence electrons to another atom, usually a nonmetal, to form an ionic bond. The electron exchange results in an ionic bond formed by the electrostatic attraction between the two atoms.
We can say that an anion is an atom that receives electrons and becomes negatively charged, whereas a cation is an atom that loses one or more valence electrons to become a positively charged ion. Ionic bonds are formed when the valence electrons of two atoms, typically metal and a nonmetal, exchange. By losing or gaining valence electrons, ions can obey the octet rule and become more stable. The vast majority of ionic substances are non-reactive. As a result, ions join in such a way that their charges are neutralized.
Generally, ionic bonding is the complete transfer of valence electron(s) between atoms. It’s a type of chemical bond that results in the formation of two ions with opposing charges. In ionic bonding, the metal loses electrons to become a positively charged cation, while the nonmetal receives those electrons to become a negatively charged anion.
The ionic bond formation is dependent on two factors:

The positive and negative charges of the oppositely charged ions attract each other, forming an ionic combination. Ionic bonds can also form when two atoms have a substantial electronegativity difference. This discrepancy results in uneven electron sharing, in which one atom entirely loses one or more electrons while the other atom receives one or more electrons, as in the formation of an ionic bond between a metal atom (sodium) and a nonmetal atom (fluorine).
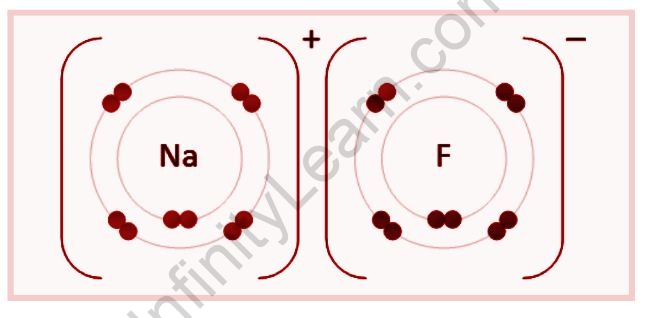
Ionic compounds are formed when electrons transfer from metal to nonmetal. As per Duplet or Octet rule, an ionic compound is generated when atoms of metals from groups 1 to 3 in the periodic chart lose electrons to atoms of nonmetals from groups 5 to 7 in the periodic table to complete their stable electronic configuration. The protons of these atoms remain unchanged during these electron exchanges.
Metals lose electrons to complete their octet in a reaction between metals and nonmetals, while nonmetals gain electrons to complete their octet. The structure of an ionic compound is determined by the relative sizes of the cations and anions. Salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds are ionic compounds. All the ionic solids are held together by the electrostatic interaction of positive and negative ions.
The ionic bonds between the charged particles from a massive ion structure. Because the ions are securely bound in these massive complexes, breaking all of the connections takes a lot of energy. And finally, it is clear that ionic compounds have high melting and boiling points. The interaction between magnesium with chlorine is an example of ionic compounds.

Because the sugar solution is a covalent rather than an ionic compound, it contains just molecules rather than ions. As a result, the sugar solution is unable to conduct electricity.
Because the oppositely charged ions in Sodium Chloride are locked together by a strong electrostatic force of attraction, there are no free ions to convey electric current.
Because the electrostatic forces of attraction are overcome by heat energy, molten sodium chloride conducts electricity. As a result, the ions are no longer bound and carry electric currents.